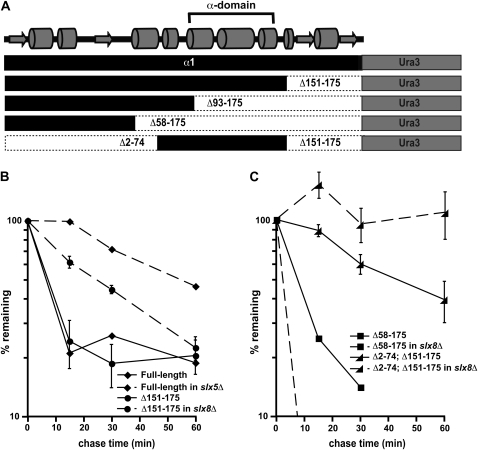
Figure 8.—
The α-domain acts as a Slx5–Slx8-dependent degradation signal. (A) Diagram of the α1-Ura3 fusion proteins analyzed for this study. Shown at the top is a predicted secondary structure of α1, including the position of the conserved α-domain. (B) Cycloheximide-chase analysis of full-length α1-Ura3 turnover at 30° in wild-type and slx5Δ cells and the Δ151–175 derivative of α1-Ura3 in wild-type and slx8Δ cells. (C) Cycloheximide-chase analysis of the Δ58–175 derivative of α1-Ura3 turnover at 30° in wild-type and slx8Δ cells and the Δ2–74; Δ151–175 derivative of α1-Ura3 in wild-type and slx8Δ cells. Note that the results of experiments with the Δ93–175 derivative of α1-Ura3 are not shown because this protein is undetectable on immunoblots of wild-type and slx8Δ cells, suggesting that the Δ93–175-Ura3 protein is very unstable in both of these strains. This protein fusion is expressed in wild-type cells, since radiolabeled protein can be detected by pulse labeling cells with Tran35S-Label. Pulse-chase analysis at 30° measuring the stability of the Δ93–175-Ura3 protein indicate a half-life of ∼2 min.