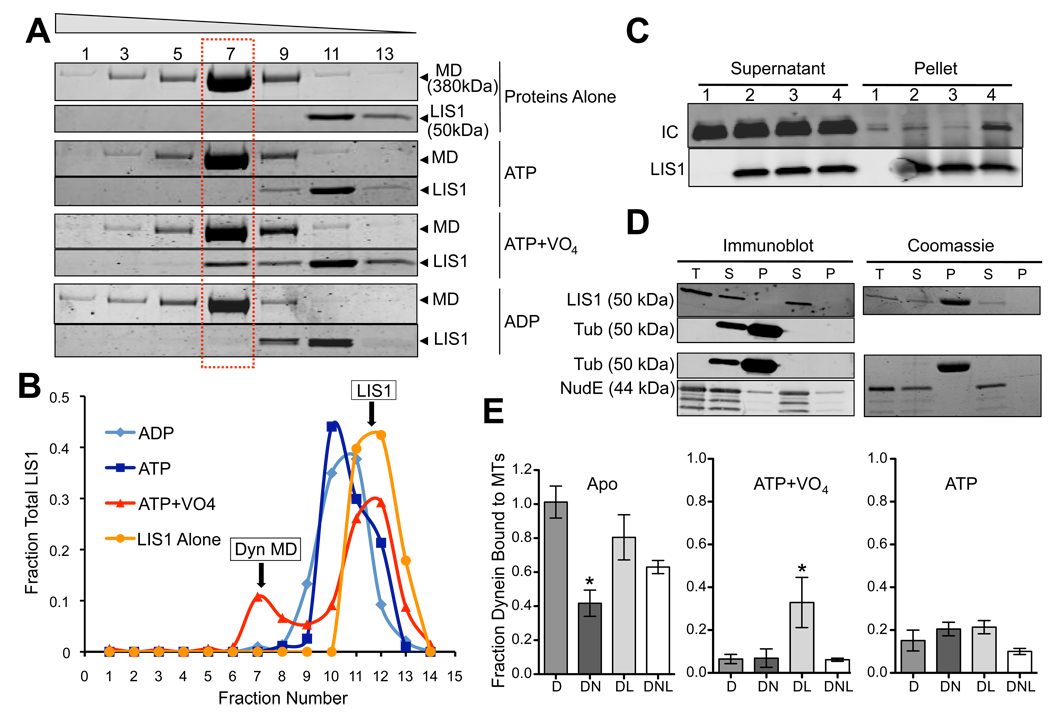
Fig. 2. Effect of Nucleotides on Dynein-NudE-LIS1 interactions.
(A) Effect of nucleotides on LIS1 binding to dynein motor domain. Purified baculovirus-expressed dynein motor domain (MD) and LIS1 were incubated separately or together and sedimented through sucrose gradients containing the indicated nucleotides. Coomassie brilliant blue stained gels show a fraction of LIS1 to co-sediment with the purified dynein motor domain peak (dashed box) in the presence of ATP and VO4, but not ATP alone or ADP. Fraction numbers are indicated at top. (B) Quantitation of LIS1 distribution showing a major low s-value peak for the free protein, and, in the ATP+VO4 condition, a smaller peak cosedimenting with the dynein motor domain. (C) Effect of nucleotides on LIS1 binding to purified calf brain dynein. Dynein was incubated with beads alone (lane 1) or LIS1-coated beads in the absence of nucleotide (lane 2) or in the presence of ATP (lane 3) or ATP+VO4 (lane 4). Dynein (IC) is enriched in the LIS1 pellet only in the ATP+VO4 condition. (D) LIS1 and NudE do not bind to microtubules. Purified LIS1 and NudE were sedimented in the presence or absence of microtubules. Total protein (T), supernatants (S) and pellets (P) are shown by immunoblotting and Coomassie blue staining. LIS1 is obscured by tubulin in the Coomassie blue-stained gel, but immunoblotting shows LIS1 not to sediment with microtubules. NudE also shows no evidence of microtubule cosedimentation. (E) Effect of LIS1 and NudE on dynein binding to microtubules. Purified brain dynein was mixed with microtubules in the absence of nucleotide (Apo) or in the presence of ATP or ATP+VO4, and the microtubules were sedimented. NudE strongly inhibited binding of dynein to microtubules in the apo state (DN; *P = 0.0011 two-tailed t-test), an effect partially rescued by LIS1 (DNL). Conversely, LIS1 increased dynein binding to microtubules in the ATP+VO4 state by approximately 5-fold (DL; * P = 0.0131 two-tailed t-test). Error bars represent mean +/− SD of 3 experiments.