Abstract
Nitric oxide (NO) is a gas with crucial signaling functions in plant defense and development. As demonstrated by generating a triple nia1nia2noa1-2 mutant with extremely low levels of NO (February 2010 issue of Plant Physiology), NO is synthesized in plants through mainly two different pathways involving nitrate reductase (NR/NIA) and NO Associated 1 (AtNOA1) proteins. Depletion of basal NO levels leads to a priming of ABA-triggered responses that causes hypersensitivity to this hormone and results in enhanced seed dormancy and decreased seed germination and seedling establishment in the triple mutant. NO produced under non-stressed conditions represses inhibition of seed developmental transitions by ABA. Moreover, NO plays a positive role in post-germinative vegetative development and also exerts a critical control of ABA-related functions on stomata closure. The triple nia1nia2noa1-2 mutant is hypersensitive to ABA in stomatal closure thus resulting in a extreme phenotype of resistance to drought. In the light of the recent discovery of PYR/PYL/RCAR as a family of potential ABA receptors, regulation of ABA sensitivity by NO may be exerted either directly on ABA receptors or on downstream signalling components; both two aspects that deserve our present and future attention.
Key words: nitric oxide, abscisic acid, seed germination, stomata opening
Plant development is the result of the succesfull execution of several programs that control the transition between different growth phases. Every developmental transition is regulated through coordinated mechanisms that involved exogenous environmental factors such as light and temperature as well as endogenous cues, including levels of primary and secondary metabolites. Among the latter, hormones such as gibberellins (GA), auxins, citokinins, ethylene and abscisic acid (ABA) participate in the control of most of the developmental transitions.1,2 During the last years, nitric oxide (NO) has gained an increasing role as an essential player in plant defense responses3 as well as a co-regulator of many developmental processes.4 However, studies of NO function as a regulatory molecule in plants have been hampered by the scanty, limited and controversial knowledge on how this gas is synthesized in plants.5,6 This situation has moved researchers in this area to adopt pharmacological approaches based on chemicals acting as artificial NO donors as well as inhibitors or scavengers of NO action. The lack of specificity and the inherent artificial effects of these chemicals can be overcome by genetic approaches based on the use of mutants with endogenous low levels of NO. In February 2010 issue of Plant Physiology, we report the generation and further characterization of a triple nia1nia2noa1-2 mutant that contains extremely low levels of NO due to the impairment of two NO biosynthetic pathways involving nitrate reductase (NIA/NR) or NO Associated 1 (AtNOA1) proteins.7 These findings support that NO is mainly produced through those pathways in Arabidopsis. However, the possible existence of a minor still uncharacterized pathways involved in the residual production of NO can not be ruled out at this time.
Further functional characterization of nia1nia2noa1-2 mutant in terms of development has pointed to NO as an overall positive regulator of plant growth, affecting to almost every developmental stage from seed germination to reproductive development. Accordingly, triple mutant plants display a delayed growth resulting in small shoot and root size and they also produce low amounts of viable seeds.7
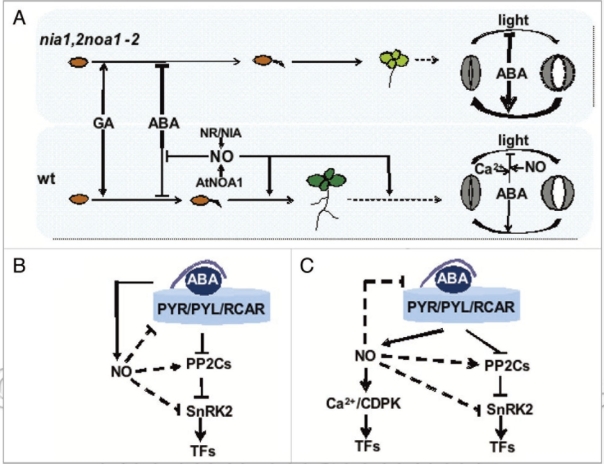
Dormancy and seed germination are developmental programs largely regulated by the combined action of GA and ABA.1 GA promote breaking of dormancy and promote germination whereas ABA acts as a brake in those processes, thus ensuring a timely seed germination. Our data from the characterization of dormancy and seed germination in the nia1nia2noa1-2 mutant suggest that NO’s role in the control of those processes may be exerted through modulation of the sensitivity to ABA (Fig. 1A). Seeds from NO deficient plants have increased dormancy and lower seed germination and seedling establishment rates than wild type seeds due to the enhanced ABA inhibitory action. These effects can be reversed by exogenous application of NO to nia1nia2noa1-2, suggesting that the sensitivity to ABA is actually controlled by the endogenous levels of NO. The recent identification of PYR/PYL/RCAR family of ABA receptors,8,9 and the further characterization of the essential ABA regulatory module including receptor, protein phosphatases of the 2C class and kinases of the SnRK2 family10 point to these components as potential targets of NO in regulating sensitivity to ABA (Fig. 1B). This work is in progress in our lab but we already know that some of the PYR/PYL/RCAR receptors and SnRKs are actually regulated by NO and also that this regulation may be exerted at different levels (Lozano-Juste J and León J, unpublished data).
Figure 1.
Interactions between NO and ABA results in modulated sensitivity to ABA throughout development. (A) NO synthesized through nitrate reductase (NR/NIA) and NO associatedI (AtNOA1) protein regulate germinative and post-germinative development as well as stomata movements through modulation of the sensitivity to ABA. Arrows and bars represent positive and negative effects, and the thickness of lines are proportional to the magnitud of regulatory effects. (B) Scheme of a minimal ABA signalling module and the potential targets of NO. Dashed lines represent effects still to be demonstrated. (C) ABA signalling in stomata guard cells through Ca2+-dependent and -independent pathways and the potential interactions with NO as represented by dashed lines.
The enhanced sensitivity to ABA observed in germinative and post-germinative development of nia1nia2noa1-2, is extended throughout plant life cycle and it is actually the cause of the very strong resistance of nia1nia2noa1-2 plants to water deficit conditions.7 Stomatal aperture is a fine-tuned process controlled mainly through a balance between the light-promoted opening and the ABA-mediated promotion of closure and inhibition of opening11 (Fig. 1A). It has been previously reported that ABA function on stomata movements involve the participation of NO as well as Ca2+ in such a way that Ca2+ chelators and NO scavengers block ABA action on stomata movements.12 Stomata of nia1nia2noa1-2 leaves, despite of being depleted of NO, are not impaired for ABA inhibition of stomata opening but, in turn, they seem to be primed for a more efficient ABA response (Fig. 1A). Contrary to the Ca2+ requirements for ABA action on wild type stomata movements, this process is not affected by Ca2+ chelators in nia1nia2noa1-2 stomata, and it thus seems to be independent of Ca2+ in NO-deficient backgrounds (Fig. 1C). As mentioned above, NO might regulate sensitivity to ABA by acting on ABA receptors or on SnRKs, some of which are Ca2+-independent kinases. Both receptors and Ca2+--independent kinases are likely targets of NO in the modulation of stomata sensitivity to ABA thus explaining the more efficient stomata closure in nia1nia2noa1-2 leaves, and the consequent low rates of evapotranspiration that leads to the extreme resistance of triple mutant plants to drought.
The future characterization of the interactions between NO and key components of ABA signaling will be the basis for a better knowledge of the functional interactions between different hormones in plant development and defense, but it will also open up new possibilities of identifying new targets and strategies leading to improved drought resistance.
Addendum to: Lozano-Juste J, León J. Enhanced abscisic acid-mediated responses in nia1nia2noa1-2 triple mutant impaired in NIA/NR- and AtNOA1-mediated nitric oxide biosynthesis in Arabidopsis. Plant Physiol. 2009 doi: 10.1104/pp.109.148023.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11235
References
- 1.Holdsworth MJ, Bentsink L, Soppe WJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 2.Davis SJ. Integrating hormones into the floraltransition pathway of Arabidopsis thaliana. Plant Cell Environ. 2009;32:1201–1210. doi: 10.1111/j.1365-3040.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 3.Leitner M, Vandelle E, Gaupels F, Bellin D, Delledonne M. NO signals in the haze: nitric oxide signalling in plant defense. Curr Opin Plant Biol. 2009;12:451–458. doi: 10.1016/j.pbi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 4.López-Bucio J, Acevedo-Hernández G, Ramírez-Chávez E, Molina-Torres J, Herrera-Estrella L. Novel signals for plant development. Curr Opin Plant Biol. 2006;9:523–529. doi: 10.1016/j.pbi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Wilson ID, Neill SJ, Hancock JT. Nitric oxide synthesis and signalling in plants. Plant Cell Environ. 2008;31:622–631. doi: 10.1111/j.1365-3040.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 6.Moreau M, Lindermayr C, Durner J, Klessig DF. NO synthesis and signaling in plants—where do we stand? Physiol Plant. 2009;138:372–383. doi: 10.1111/j.1399-3054.2009.01308.x. DOI: 10.1111/j.1399-3054.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- 7.Lozano-Juste J, León J. Enhanced abscisic acidmediated responses in nia1nia2noa1-2 triple mutant impaired in NIA/NR- and AtNOA1-mediated nitric oxide biosíntesis in Arabidopsis. Plant Physiol. 152:891–903. doi: 10.1104/pp.109.148023. doi 10.1104 pp.109.148023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 9.Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, et al. In vitro reconstitution of an abscisic acid signalling pathway. Nature. 2009;462:660–664. doi: 10.1038/nature08599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roelfsema MR, Hedrich R. In the light of stomatal opening: new insights into ‘the Watergate’. New Phytol. 2005;167:665–691. doi: 10.1111/j.1469-8137.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- 12.García-Mata C, Lamattina L. Abscisic acid (ABA) inhibits light-induced stomatal opening through calcium- and nitric oxide-mediated signaling pathways. Nitric Oxide. 2007;17:143–151. doi: 10.1016/j.niox.2007.08.001. [DOI] [PubMed] [Google Scholar]