Abstract
Background. In patients with unstable angina or non-ST-elevation acute coronary syndrome (NSTE-ACS) who are eligible for PCI, routine stenting is the recommended treatment strategy, based on the opinion of experts. Provisional stenting may provide a viable alternative by retaining the early benefits of stenting without its potential late hazards.
Method. Patients with NSTE-ACS were randomised to provisional or routine stenting after coronary angiography. Patients were followed for up to ten years. The occurrence of major adverse cardiac events (MACE) was recorded.
Results. 237 consecutive patients with NSTE-ACS were randomly assigned to routine stenting (n=116) or provisional stenting (n=121). No difference in the incidence of MACE at 30 days was observed. At six months, angiographic restenosis was lower in the routine stenting group (41 vs. 20%, p=0.02), paralleled by more MACE in the provisional stenting group at one year (40.5 vs. 27.6%, p=0.036). At complete follow-up the difference in MACE was not significant (61.2 vs. 50%, p=0.084) because of relatively more target lesion revascularisations in the routine stent group. There was no difference in the incidence of very late stent thrombosis (1.7 vs. 3.4%, p=0.439). The only independent predictor of MACE was β-blocker use (RR 0.62 [0.431; 0.892] p=0.010).
Conclusion. In selective patients with NSTE-ACS, routine stenting was more beneficial than provisional stenting for a period of up to five years, driven by a reduction in repeat revascularisation procedures. After this period, the benefit was no longer significant. Beta-blocker use was the only independent predictor of MACE throughout the complete follow-up period. (Neth Heart J 2010;18:307–13.)
Keywords: Angioplasty, Acute Coronary Syndrome, Stents, Time Factors
The short- and long-term benefits of stenting in stable coronary artery disease have been well established in randomised clinical trials.1-4 In comparison with balloon angioplasty, stenting has proven to reduce the rates of acute vessel closure and restenosis at four to eight months. These advantages and favourable outcome of routine stenting in unselected patient populations5 have resulted in a wide use of stents.6
Therefore, in patients with unstable angina or non-ST-elevation acute coronary syndrome (NSTE-ACS) who are eligible for PCI, routine stenting is the recommended treatment strategy.7 Since randomised trials are lacking, this recommendation is based solely on the opinion of experts. However, in NSTE-ACS, inflammation,8,9 a prothrombogenic state10,11 and positive remodelling12,13 may attenuate the clinical outcome after stenting. A strategy of provisional stenting in NSTE-ACS patients may provide a viable alternative.
In this study, the short- and long-term (up to ten years) outcome after routine stenting for NSTE-ACS was compared with provisional stenting in a randomised design.
Patients and methods
Patients
Patients with chest pain at rest and documented ECG changes within the preceding 48 hours were candidates for the study (Braunwald class 3B or C). Angiographic inclusion criteria were an original culprit lesion in a native coronary artery suitable for stenting, lesion length <30 mm and a vessel diameter >3 mm (visual estimate). Exclusion criteria were inability to give informed consent, participation in another study and life expectancy of <1 year.
Angiographic exclusion criteria were unprotected left main disease or severe multivessel disease necessitating urgent bypass surgery, target lesion located in a bifurcation with a large side branch, and excessive proximal vessel tortuosity. Patients were randomised by means of a closed envelope system.
Procedure
A strategy of culprit lesion angioplasty was followed. Pretreatment with glycoprotein IIb/IIIa inhibitors or intravascular ultrasound was not used. Initially, bare Palmaz-Schatz (Cordis®) stents were used. Primary stenting without predilatation was not performed. In patients assigned to provisional stenting, prolonged balloon inflations had to be attempted before stenting was considered. Quantitative coronary angiography (QCA) was analysed by an independent core laboratory (Diagram, Zwolle, the Netherlands).
Periprocedural regimen
The initial post-stenting regimen consisted of heparin infusion started two hours after sheath removal. Warfarin was given for at least three months and aspirin (80 mg/day) indefinitely. After January 1996, a regimen of ticlopidine (250 mg/day for at least two weeks) and aspirin was used. Heparin infusion (1 mg/kg/h) or subcutaneous low-molecular-weight heparin (0.6 ml twice daily) was given for 48 hours after sheath removal in all patients.
Definitions
Angiographic success was defined as a reduction of the target lesion to less than 50%. Procedural success was defined as angiographic success without the occurrence of a procedure-related adverse event.
Myocardial infarction was defined as chest pain with ST-T segment changes and an increase in creatine kinase levels of more than twice the upper limit of normal or an increase over the previous value if the level had not dropped below the upper limit of normal.
If coronary angiography was not performed, the electrocardiogram was used to identify the most probable infarct-related artery.
Sudden cardiac death was defined as unexpected death without a documented non-cardiac explanation. For all stented patients, the Academic Research Consortium (ARC) definitions of stent thrombosis were used.14
Endpoints
The primary endpoint of the study was the occurrence of MACE: death, myocardial infarction, CABG or PCI at 30 days, 12 months and at long-term follow-up (for up to ten years). The indication for a second intervention had to be substantiated by symptoms and/or electrocardiographic or scintigraphic evidence of ischaemia.
The angiographic endpoint was defined by the incidence of angiographic restenosis (diameter stenosis of >50%) as measured by QCA at six months follow-up. In retrospect the occurrence of stent thrombosis was assessed.
Follow-up
In February 2001 and between April and June 2005, patients were contacted for a structured telephone interview. If data were not available, the last known date of follow-up was used.
Statistical analysis
Data were analysed by use of comparison between groups using the intention-to-treat principle. Continuous variables are expressed as mean ±SD and compared by use of Student’s t-test, whereas discrete variables are given as absolute values and percentages. The Χ2 test was used to compare proportions, or the Fisher’s exact test where appropriate.
The difference in event rates between groups during follow-up was assessed by the Kaplan-Meier method, using the log-rank test. Multivariate analysis was performed using the Cox proportional hazard method, permitting calculation of odds ratios that may be interpreted as relative risks with 95% confidence intervals. All statistical tests were performed two-sided, with a p value of <0.05 considered as statistically significant.
Results
Patient characteristics
Between January 1995 and September 1998, 237 patients were randomised to provisional stenting (n=121) or routine stenting (n=116). Baseline characteristics are given in table 1. Patients allocated to provisional or routine stenting were mainly comparable, except for hypertension and aspirin use at entry and use of β-blockers and coumadin and ticlopidine at discharge.
Table 1.
Baseline characteristics.
| Provisional (n=121) | Routine (n=116) | P value | |
|---|---|---|---|
| Age | 59±10 | 59±11 | 0.991 |
| Gender | 88 (73) | 83 (72) | 0.952 |
| Number of patients with | |||
| - Refractory angina <48 hrs | 88 (73) | 79 (68) | |
| - Angina after recent AMI | 25 (21 ) | 29 (25) | 0.713 |
| - Stabilised angina | 8 (6.6) | 8 (6.9) | |
| Previous history | |||
| - Myocardial infarction | 40 (33) | 42 (36) | 0.610 |
| - PCI | 13 (11) | 13 (11) | 0.909 |
| - CABG | 4 (3.3 ) | 6 (5.2) | 0.475 |
| Risk factors | |||
| - Diabetes mellitus | 10 (8.3 ) | 11 (9.5) | 0.741 |
| - Hypercholesterolaemia | 49 (41) | 51 (44) | 0.589 |
| - Hypertension | 54 (45) | 32 (28) | 0.006 |
| - Smoking | 54 (45) | 58 (50) | 0.408 |
| - Family history | 61 (50) | 63 (54) | 0.548 |
| Medication at entry | |||
| - Beta-blockers | 87 (73) | 84 (72) | 0.988 |
| - Calcium antagonist | 34 (28) | 40 (35) | 0.309 |
| - Nitrates | 107 (89) | 105 (91) | 0.731 |
| - Heparin | 93 (78 ) | 84 (72)97 (84) | 0.235 |
| - Aspirin | 106 (88) | 112 (97) | 0.017 |
| Medication at discharge | |||
| - Beta-blockers | 97 (82) | 78 (69) | 0.027 |
| - Calcium antagonist | 29 (24) | 40 (35) | 0.066 |
| - Nitrates | 30 (25) | 21 (19) | 0.223 |
| - Aspirin | 115 (97) | 109 (97) | 0.941 |
| - Ticlopidine | 24 (20) | 89 (79) | <0.001 |
| >- Coumadins | 3 (2.5) | 17 (15) | 0.001 |
| Coronary angiography: | |||
| Vessel disease | |||
| - 1 | 72 (60) | 79 (68) | |
| - 2 | 39 (32) | 28 (24) | 0.354 |
| - 3 | 10 (8) | 9 (8) | |
| Culprit lesion | |||
| - RCA | 44 (36) | 39 (34) | |
| - LAD | 58 (49) | 55 (47) | 0.781 |
| - RCX | 19 (15) | 22 (19) |
Data are given as n (%) or mean ± standard deviation. AMI=acute myocardial infarction, PCI=percutaneous coronary intervention, CABG=coronary artery bypass graft, RCA=right coronary artery, LAD=left anterior descending artery, RCX=right circumflex artery.
Procedure
QCA of the index procedure was performed in 230 patients (97%). Vessel size at QCA was more than 3 mm in most patients. However, a considerable proportion of patients had a reference diameter of less than 3 mm (provisional stenting 39%, routine stenting 45%; p=0.371).
In the routine stent group, a stent could be implanted in all patients. In 20% of these patients more than one stent was inserted. A Palmaz-Schatz stent (Cordis®) was used in 63%, Crossflex (Cordis®) in 19%, and another type of stent in 18%: AVE (Medtronic®), Crown (Cordis®) and NIR (Boston Scientific®).
In the provisional stent group,19% had a stent implanted, mostly because of dissection. A larger immediate gain after stenting resulted in a larger minimal luminal diameter (MLD) and smaller residual diameter stenosis. The resulting angiographic success was 96% after provisional stenting and 98% after routine stenting. Procedural success was 88% after routine stenting and 84% after provisional stenting. Follow-up angiography was performed after 234±89 days in 191 patients (81%). Despite a larger late loss after stenting, MLD was still larger after routine stenting in comparison with provisional stenting at six months, resulting in a 50% risk reduction (RR 0.50, 95% CI 0.31 to 0.80) in restenosis rate.
Short- and mid-term follow-up
About 5% of the included NSTE-ACS patients had a non-ST-elevation myocardial infarction before randomisation. In the routine stenting group, three patients experienced a subacute stent thrombosis, within two hours after the procedure. There was no significant difference in the incidence of repeat percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG) or the cumulative incidence of MACE up to 30 days (table 2).
Also, no difference in the incidence of clinically important bleeding between routinely and provisionally stented patients was observed (4.3 vs. 3.3% respectively, p=0.69). MACE at one year were more frequent in the provisional stenting group, (40.5 vs. 27.6% p=0.036, table 2), because of more clinically driven revascularisation procedures, particularly more CABG. Although target lesion revascularisations (TLR) were more frequent in the provisional stenting group, a considerable number of these interventions were due to non-target-related lesions. At one year of follow-up, 81 cardiac events (61% of total MACE during complete follow-up) had occurred. During the period of one to five years, event rates were comparable between the two treatment groups. Myocardial infarction occurred in 2.5% of patients in both groups and 13 patients died. Cardiac death occurred in three patients in the provisional stent group and in one patient in the routine stent group. There were no differences in TLR. At five-year follow-up, there was still a significant difference in MACE between both treatment groups.
Table 2.
Clinical events for up to ten years.
| Provisional (n=121) | Routine (n=116) | P value | |
|---|---|---|---|
| Up to 30 days | |||
| Death | 0 (0) | 1 (0.9) | 0.489 |
| MI | 16 (13.2) | 12 (10.3) | 0.493 |
| PCI | 6 (5.0) | 4 (3.4) | 0.749 |
| CABG | 3 (2.5) | 3 (2.6) | 1.000 |
| TLR | 7 (5.8) | 6 (5.2) | 0.836 |
| Total MACE at 30 days | 21 (17.4) | 14 (12.1) | 0.252 |
| 30 days to 1 year | |||
| Death | 0 (0) | 1 (0.9) | 0.489 |
| MI | 2 (1.7) | 2 (1.7) | 1.000 |
| PCI | 25 (20.7) | 19 (16.4) | 0.397 |
| CABG | 9 (7.4) | 3 (2.6) | 0.089 |
| TLR | 21 (17.4) | 14 (12.1) | 0.252 |
| MACE 30 days to 1 year | 35 (28.9) | 22 (19.0) | 0.073 |
| Total MACE at 1 year | 49 (40.5) | 32 (27.6) | 0.036 |
| 1 year to 5 years | |||
| Death | 9 (7.4) | 4 (3.4) | 0.178 |
| MI | 3 (2.5) | 3 (2.6) | 1.000 |
| PCI | 10 (8.3) | 13 (11.2) | 0.444 |
| CABG | 6 (6.6) | 6 (5.2) | 1.000 |
| TLR | 7 (5.7) | 8 (6.9) | 0.725 |
| MACE 1 to 5 years | 15 (12.4) | 19 (16.4) | 0.382 |
| Total MACE at 5 years | 65 (53.7) | 46 (39.7) | 0.030 |
| 5 years to 10 years | |||
| Death | 9 (7.4) | 7 (6.0) | 0.667 |
| MI | 0 | 2 (1.7) | 0.239 |
| PCI | 5 (4.1) | 9 (7.8) | 0.237 |
| CABG | 1 (0.8) | 3 (2.6) | 0.361 |
| TLR | 2 (1.7) | 6 (5.2) | 0.133 |
| MACE 5-10 years | 15 (12.4) | 19 (16.4) | 0.382 |
| Total MACE at 10 years | 74 (61.2) | 58 (50.0) | 0.084 |
Data are given as n (%). MI=acute myocardial infarction, PCI=percutaneous coronary intervention, CABG=coronary artery bypass graft, TLR=target lesion revascularisation, MACE=major adverse cardiac events.
Complete follow-up
Complete follow-up was available in 224 patients (97%), with a mean period of 7.9±1.9 years. In this period 30 patients died, 18 in the provisional stenting group versus 12 in the routine stenting group (p=0.33). The frequency of cardiac death was similar for both groups: two-thirds of deaths had a non-cardiac cause, mostly malignancies (42%). Documented myocardial infarction after 30 days was a relatively rare event and occurred in 12 patients (table 3).
Table 3.
Irreversible endpoints, 30 days to ten years of follow-up.
| Provisional (n=121) | Routine (n= 116) | P value | |
|---|---|---|---|
| MI | 5 (4.1) | 7 (6.0) | 0.564 |
| - Q wave | 3 (2.5) | 4 (3.4) | 0.717 |
| - TVR | 2 (1.7) | 4 (3.4) | 0.439 |
| - CK-MB >50 | 2 (1.7) | 4 (3.4) | 0.439 |
| - Anterior | 0 | 1 (0.9) | 0.489 |
| - Definite very late ST | 0 | 1 (0.9) | 0.489 |
| - Probable very late ST | 1 (0.8) | 1 (0.9) | 0.976 |
| Death | 18 (14.9) | 12 (10.3) | 0.333 |
| - Cardiac | 5 (4.1) | 4 (3.44) | 1.000 |
| - Sudden cardiac death | 3 (2.5) | 2 (1.7) | 0.962 |
| - Possible very late ST | 1 (0.8) | 2 (1.7) | 0.616 |
| - CVA | 2 (1.7) | 0 | 0.498 |
| - Malignancy | 10 (8.2) | 3 (2.5) | 0.084 |
| - Pulmonary disease | 0 | 2 (1.7) | 0.239 |
| - Renal failure | 1 (0.8) | 1 (0.9) | 0.976 |
| - Other | 0 | 2 (1.7) | 0.239 |
Data are given as n (%). MI=myocardial infarction, TVR=target vessel-related; ST=stent thrombosis, CVA-cerebrovascular accident.
At complete follow-up, possible, probable or definite very late stent thrombosis was recorded in two patients in the provisionally stented group versus four patients in the routinely stented group (1.7 vs. 3.4%; p= 0.439). In figure 1, death or myocardial infarction free survival is depicted. There was no difference between the two treatment groups throughout the study period. In figure 2, the survival free of TLR, including both CABG and PCI, is depicted. It is shown that in the first year of follow-up the benefit of stenting is established. After five years, there is a relative increase in repeat interventions in the routinely stented group. The total incidence of MACE at ten years of follow-up was 19% lower in the routine stent group (50 vs. 61%) but without achieving statistical significance (p=0.084). MACE-free survival for up to ten years is depicted in figure 3. After univariate and multivariate analysis, β-blocker use at discharge was the only independent predictor of MACE for up to ten years.
Figure 1.
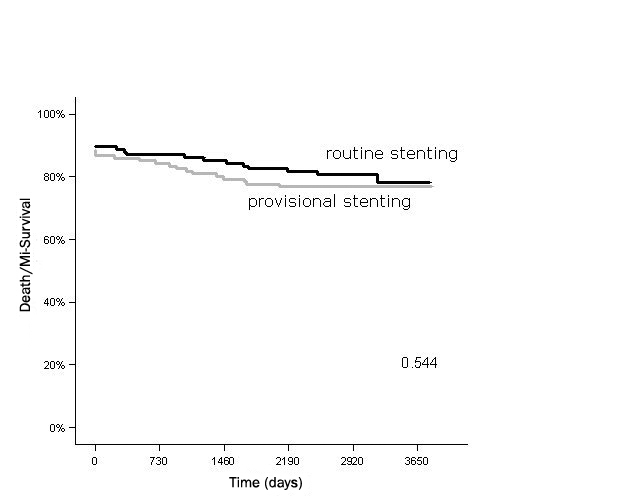
Death/myocardial infarction (MI) free survival for up to ten years.
Figure 2.
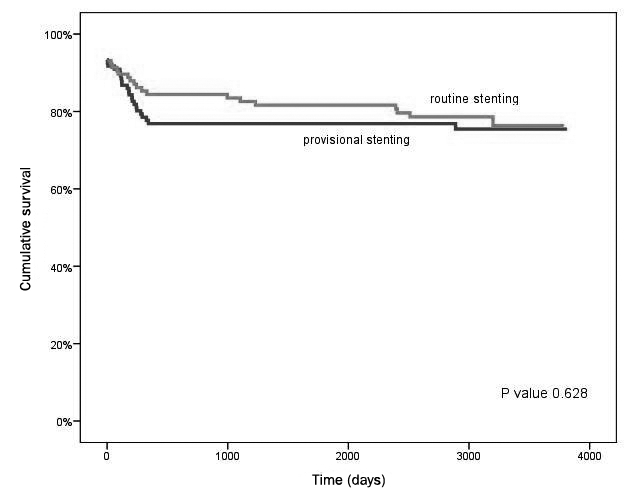
TLR-free survival for up to ten years.
Figure 3.
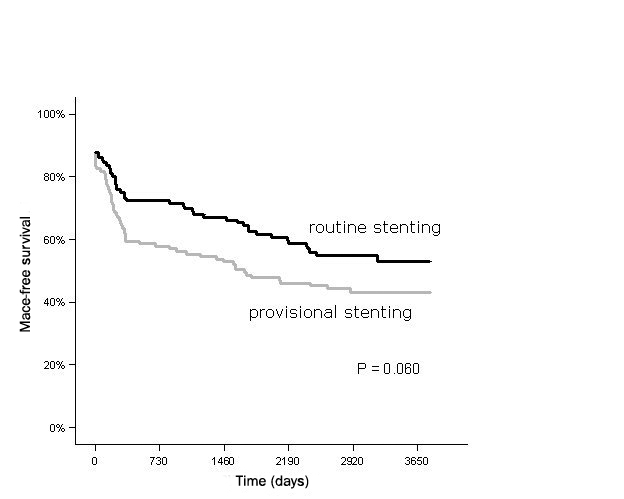
MACE-free survival for up to ten years.
Discussion
In this randomised trial we found no differences in the incidence of death or myocardial infarction between the two treatment strategies throughout the follow-up period of up to ten years. However, routine stenting did reduce the restenosis rate and the need for additional revascularisation procedures, particularly in the first year.
Short-term outcome
Several studies have underlined the short-term safety and efficacy of stenting in unstable coronary artery disease and demonstrated improved outcome with concomitant administration of combined platelet therapy or glycoprotein IIb/IIIa blockers.15-17 However, the impact of routine stenting in relation to provisional stenting in NSTE-ACS is not well established. In our study stenting provided additional safety by preventing CABG or incomplete index segment revascularisation in 19% of the patients in the provisional stenting group.
Long-term outcome
Routine stenting was beneficial in the first five years of follow-up. This benefit was entirely driven by a lower number of revascularisation procedures. This observation is in line with previous studies that investigated both stable and unstable patients, showing a sustained decrease in TLR in the routine stenting group, without any differences in the incidence of survival or myocardial infarction18,19 and a low annual hazard rate of TLR after the first year of follow-up.20 In this trial, the early benefit of reducing TLR is gradually lost. Although the unstable presentation at baseline could be responsible, differences between the two treatment groups are too small to support this assumption. It is more likely that the nonsignificant long-term outcome results from general progression of coronary artery disease in treated and nontreated segments.
It is important to emphasise that cardiac death and myocardial infarction were relatively uncommon events. Although late stent thrombosis was not prospectively defined as an endpoint, in retrospect low incidences of stent thrombosis were found. Therefore, in our study population of NSTE-ACS patients, both provisional and routine bare metal stenting appeared safe treatment strategies at long-term follow-up. These findings have also been reported in a previous randomised trial in STEMI patients.21 Finally, using multivariate analyses, randomisation to routine stenting had no significant protective effect for the incidence of MACE over the complete follow-up period. The only independent predictor was β-blocker use at discharge. This finding is in line with previous trials that have studied the long-term benefit of β-blocker use on the occurrence of MACE.22
Implications
In this study, there was a considerable discrepancy between the 50% reduction in angiographic restenosis rate and nonsignificant long-term clinical benefit after routine stenting. This implies that angiographic endpoints may be suboptimal surrogates for long-term clinical results in an NSTE-ACS population. Furthermore, most benefit from routine stenting seems to be confined to the first year after treatment. Bioabsorbable stents that dissolve after one year may provide the best of both treatment strategies and deserve further study. Finally, the importance of adequate medical therapy including the use of a β-blocker can not be overstated in this population.
Limitations
The most important limitation of this trial is the small sample size. Furthermore, randomisation was performed after coronary angiography. Randomisation before angiography would have attenuated the results of stenting because implanting a stent would not have been possible in some patients with small vessels and diffuse disease. However, a significant proportion of the patients had a reference diameter <3 mm at QCA. At the time this trial was initiated, today’s optimal pharmacological therapy with clopidogrel, glycoprotein IIb/IIIa blockers and statins was not available. Of course, this is inherent to a study with (very) long-term follow-up. Furthermore, the number of myocardial infarctions could be underestimated according to the current definition of myocardial infarction.23 Finally, long-term follow-up by telephonic interviews can be considered as retrospective and was not prespecified.
Conclusion
Routine stenting in selective patients with NSTE-ACS is beneficial due to a reduction in additional revascularisation procedures, mostly achieved within the first year. During a follow-up period of up to ten years, this effect is gradually lost, probably as a result of ongoing coronary disease, rather than as a result of unstable disease at baseline. Furthermore, this study reveals that the incidence of stent thrombosis is low when bare metal stents are used and underlines the beneficial effect of β-blockers on the occurrence of MACE.
References
- 1.Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable- stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. 1994;331;489-95. [DOI] [PubMed] [Google Scholar]
- 2.Kiemeneij F, Serruys PW, Macaya C, Rutsch W, Heyndrickx G, Albertsson P, et al Continued benefit of coronary stenting versus balloon angioplasty: Five-year clinical follow-up of Benestent-1 trial. J Am Coll Cardiol. 2001;37;1598-603. [DOI] [PubMed] [Google Scholar]
- 3.Betriu A, Masotti M, Serra A, Alonso J, Fernández-Avilés F, Gimeno F, et al. Randomized comparison of coronary stent implantation and balloon angioplasty in the treatment of de novo coronary artery lesions (START) A four-year follow-up. J Am Coll Cardiol. 1999;34;1498-506. [DOI] [PubMed] [Google Scholar]
- 4.Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med. 1994;331;496-501. [DOI] [PubMed] [Google Scholar]
- 5.Vernon Anderson H, Carabello BA. Provisional versus routine stenting: routine stenting is here to stay. Circulation. 2000;102;2910-4. [DOI] [PubMed] [Google Scholar]
- 6.Vernon Anderson H, Shaw RE, Brindis RG, Hewitt K, Krone RJ, Block PC, et al. A contemporary overview of percutaneous coronary interventions. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). J Am Coll Cardiol. 2002;39;1096-103. [DOI] [PubMed] [Google Scholar]
- 7.Silber S, Albertsson P, Avilés FF, Camici PG, Colombo A, Hamm C, et al. Guidelines for percutaneous coronary interventions The Task Force for Percutaneous Coronary Interventions Of the European society of Cardiology. Eur Heart J. 2005;8:804-47. [DOI] [PubMed] [Google Scholar]
- 8.Mueller Ch, Buettner HJ, Hodgson JM, Marsch S, Perruchoud AP, Roskamm H, et al. Inflammation and long-term mortality after non-ST elevation acute coronary syndrome treated with early invasive strategy in 1042 consecutive patients. Circulation. 2002;105;1412-5. [DOI] [PubMed] [Google Scholar]
- 9.Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. N Engl J Med. 2000;343;1139-47. [DOI] [PubMed] [Google Scholar]
- 10.Rioufol G, Finet G, Ginon I, André-Fouët X, Rossi R, Vialle E, et al. Multiple atherosclerotic plaque rupture in acute coronary syndrome. A three-vessel intravascular ultrasound study. Circulation. 2002;106;804-8. [DOI] [PubMed] [Google Scholar]
- 11.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the unstable plaque. Prog Cardiovasc Dis. 2002;44;349-56. [DOI] [PubMed] [Google Scholar]
- 12.Okura H, Taguchi H, Kubo T, Toda I, Yoshiyama M, Yoshikawa J, et al. Impact of arterial remodelling and plaque rupture on target and non-target lesion revascularisation after stent implantation in patients with acute coronary syndrome: an intravascular ultrasound study. Heart. 2007;93;1219-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura M, Yock PG, Bonneau HN, Kitamura K, Aizawa T, Tamai H, et al. Impact of peri-stent remodelling on restenosis: a volumetric intravascular ultrasound study. Circulation. 2001;103;2130-2. [DOI] [PubMed] [Google Scholar]
- 14.Mauri L, Hsieh W-H, Massaro J, Ho KK, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356;1020-9. [DOI] [PubMed] [Google Scholar]
- 15.Marzocchi A, Piovaccari G, Marozzini C, Ortolani P, Palmerini T, Branzi A, et al. Results of coronary stenting for unstable versus stable angina pectoris. Am J Cardiol. 1997;79;1314-8. [DOI] [PubMed] [Google Scholar]
- 16.Lincoff AM, Califf RM, Moliterno DJ, Ellis SG, Ducas J, Kramer JH, et al. Complementary clinical benefits of coronary artery stenting and blockade of platelet glycoprotein IIb/IIIa receptors. N Engl J Med. 1999;341;319-27. [DOI] [PubMed] [Google Scholar]
- 17.The EPISTENT Investigators. Randomised placebo-controlled and balloon angioplasty-controlled trials to assess safety of coronary stenting with use of platelet glycoprotein IIb/IIIa blockade. Lancet. 1998;352;87-92. [DOI] [PubMed] [Google Scholar]
- 18.Al Suwaidi J, Holmes DR, Salam AM, Lennon R, Berger PB. Impact of coronary artery stents on mortality and nonfatal myocardial infarction: Meta-analysis of randomized trials comparing a strategy of routine stenting with that of balloon angioplasty. Am Heart J. 2004;147;815-22. [DOI] [PubMed] [Google Scholar]
- 19.Kandzari DE, Tuttle RH, Zidar JP, Jollis JG. Comparison of long-term (seven year) outcomes among patients undergoing percutaneous coronary revascularization with versus without stenting. Am J Cardiol. 2006; 97;1467-72. [DOI] [PubMed] [Google Scholar]
- 20.Cutlip DE, Chhabra AG, Baim DS, Chauhan MS, Marulkar S, Massaro J, et al. Beyond Restenosis. Five-year clinical outcomes from second-generation coronary stent trials. Circulation. 2004;110;1226-30. [DOI] [PubMed] [Google Scholar]
- 21.Suryapranata H, De Luca G, van ’t Hof AWJ, Ottervanger JP, Hoorntje JCA, Dambrink JHE, et al. Is routine stenting for acute myocardial infarction superior to balloon angioplasty? A randomised comparison in a large cohort of unselected patients. Heart. 2005;91;641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson JD, Muhlestein JB, Bunch TJ, Bair TL, Horne BD, Madsen TE, et al; Intermountain Heart Collaborative Study Group. β-Blockers reduce the incidence of clinical restenosis: Prospective study of 4840 patients undergoing percutaneous coronary revascularization. Am Heart J. 2003;145;875-81. [DOI] [PubMed] [Google Scholar]
- 23.The task force for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes of the European society of cardiology. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598-660. [Google Scholar]


