Abstract
Opiates modulate nociception in vertebrates. This has also been demonstrated in a number of invertebrate models. Herein, the effect of the opiate morphine and opioid neuropeptides Endomorphin 1 and 2 on the thermal avoidance (Tav) behavior of Caenorhabditis elegans is explored. Adult wild-type C. elegans N2 were collected from NGM plates using M9 buffer and exposed to morphine and endomorphine 1 and 2 in concentrations between 10−8 and 10−4 M (2.5 pmol/mg to 25 nmol/mg) for 30 min and tested for Tav. The opioid receptor antagonists Naloxone and CTOP were tested in combination with the drugs. Forty-seven percentage of the morphine exposed worms exhibited a class I response versus 76% of the control group (P < 0.001). Endomorphin 1 and 2 also caused a statistically significant reduction in class I responses, 36 and 39%, respectively. These effects were reversed with Naloxone and CTOP. Thermonocifensive behavior in C. elegans is modulated by opioids.
Keywords: Opioid, Nematode, Nociception
Introduction
Avoidance of harmful stimuli in animals is a behavioral response important to their survival. The behavior is associated with the perception of noxious stimuli or nociception. This response in vertebrates is modulated by opiates and opioids. Opioid peptides and their receptors are common neuroendocrine system signaling molecules in vertebrates. These peptides affect a variety of physiological processes including analgesia, respiratory and cardiovascular function, and thermoregulation (Dores et al. 2002). Their presence and function in invertebrates has also been documented (Dureus et al. 1993; Duvaux-Miret et al. 1992, 1993; Dyakonova 2001; Dyakonova et al. 1999, 2000; Goumon et al. 2000; Harrison et al. 1994; Kavaliers et al. 1983; Leung et al. 1995; Pryor and Elizee 2000; Pryor et al. 2007; Renaud et al. 1995, 1996; Renzelli-Cain and Kaloustian 1995; Salzet 2001; Salzet et al. 1997; Salzet and Stefano 1997a, b; Sonetti et al. 1997; Stefano et al. 2003). Opioids play an important role in modulating nociception. The effect of morphine on the behavioral response of invertebrates to aversive stimuli is documented in the literature (Achaval et al. 2005; Barr et al. 2008; Kalil-Gaspar et al. 2007; Kavaliers et al. 1983, 1998; Lozada et al. 1988; Maldonado et al. 1989; Pryor et al. 2007; Romano et al. 1990; Romero et al. 1994). In all instances, morphine increases the latency response of the animals. Opioid receptor antagonists, e.g., naloxone and CTOP, reverse this effect. This effect of morphine on thermonocifensive behavior has also been demonstrated in the parasitic nematode Ascaris suum (Pryor et al. 2007). Evidence for the presence of opioid receptors in invertebrates has only been documented for the ganglia of the blue mussel Mytilus edulis (Cadet and Stefano 1999). Transcripts of the receptor isolated using RT–PCR show 95% sequence identity with the neuronal μ1 mammalian receptor. Expression levels of this receptor in the blue mussel are affected by temperature stress (Cadet et al. 2002). An attempt to isolate this receptor from A. suum using primers for a conserved region of the neuronal μ1 mammalian receptor was unsuccessful (Zhu et al. 2004). However, exposure of A. suum to morphine and morphine 6-glucuronide has a significant antinociceptive effect to noxious heat measured as an increase in the latency response period (Pryor et al. 2007). These results led us to undertake a similar pharmacological study using C. elegans as a closely related nematode for which substantive genome annotation has been done. While the genome of C. elegans does not appear to have ortholog genes for vertebrate opioid receptors family or their agonists, it has a very diverse repertoire of neuropeptide molecules particularly FMRFamide-related peptides (FaRPs). This family of neuropeptides is related to the opioid family in particular the pro-enkephalin precursor gene (Greenberg et al. 1983; Gupta et al. 1999).
Thermal avoidance (Tav) behavior in C. elegans has been previously reported as a thermonocifensive response to noxious heat stimuli (Wittenburg and Baumeister 1999). The molecular mechanism and neural circuit for this behavior appears to differ from that involved in thermotaxis (Julius and Basbaum 2001; Ryu and Samuel 2002). The effect of exogenous morphine and the opioids endomorphin 1 and 2 on the Tav behavior in C. elegans was explored, and the results are presented herein.
Materials and methods
Animal cultures
Stock agar cultures of wild-type N2 C. elegans were purchased from the Caenorhabditis Genetics Center (CGC) at the University of Minnesota and maintained in Nematode Growth Medium (NGM) agar plates and fed E. coli OP50 at 20–25 °C in the dark.
Behavioral assays
The worms were washed from a 3 to 4-day-old NGM plate using M9 buffer. A one hundred microliter aliquot of the wash was placed on a NGM plate without food. Adult animals were exposed to a noxious heat stimulus by using a pen with an electronically heated platinum tip (φ = 0.8 mm) (Colwood Electronics, Eatontown, NJ) mounted on a micromanipulator. The temperature of the heated platinum wire was monitored using a temperature probe (World Precision Instruments) to produce a constant radial temperature gradient at 3.0 mm from the tip of 33.0 ± 1.0°C. Exposure of the worms was done by presenting the heat stimulus at a distance of 3.0 mm from the anterior end of the animal using the micromanipulator. Once the probe reached the desired distance, the behavior was scored and the stimulus was immediately removed. Scoring of the behaviors was done using an illuminated dissecting microscope (Nova 2000, Morrell). The behavioral responses of the worms were classified according to the following categories: class 1, rapid reflexive withdrawal, backing for at least one body length followed by a heading change; class 2, rapid reflexive withdrawal but only little backing; class 3, slow backing; class 4, no response (Wittenburg and Baumeister 1999). A total of at least three replicates of 50–100 nematodes were counted for each experiment. Based on similar studies done in other invertebrates, the effect of the drug treatment is assessed as the degree of delay in the response to the noxious stimulus. In the case of Tav, a significant change in class 1 behavior was used to assess the effectiveness of the treatment.
Drug treatments
The worms were collected from the culture plates by washing with M9 buffer. One milliliter of the wash was collected into 1.5-ml centrifuge tubes. The control and drug treatments were delivered into the 1 ml wash in the appropriate volumes to render the desired final treatment concentrations. After a 30-min exposure period, a 100 μl aliquot was poured onto a NGM plate without food, and the animals were scored as explained earlier. The animals were subjected to drug treatments of morphine, endomorphine 1 (EM1) and endomorphine 2 (EM2) at concentrations between 10−8 and 10−4 M, i.e., 2.5 pmol/mg to 25 nmol/mg, for 30 min, with and without the opioid receptor antagonists naloxone and CTOP. Exposure to the antagonists was done 15 min prior to the exposure to the agonists. These concentrations are consistent with those used for morphine and naloxone in other invertebrates studies (Achaval et al. 2005; Zabala and Gomez 1991). Times and mode of exposure used were also consistent with those used for C. elegans in similar studies (Wittenburg and Baumeister 1999).
Statistical analysis
Differences between control and treatments were tested for statistical significance using a one-way ANOVA followed by multiple pair-wise comparisons of the means using the Holm-Sidak method.
Results
Worms exposed to a noxious thermal stimulus display a thermal avoidance behavior ranging from a quick retraction followed by backing up for more than one body length, class 1, a quick retraction followed by backing by less than one body length, class 2, slow backing, class 3, and no reaction, class 4. As shown in Fig. 1, 76% ± 4% (mean ± standard error) of the worms from the control group exhibited a class I behavior, 10% ± 1% belonged to class 2, 6% ± 1% to class 3, and 7% ± 4% to class 4. An antinociceptive effect is manifested at the behavioral level by a significant drop in the percentage of class 1 worms, and consequently an increase in percentage of worms in the other behavioral classes. Quantitatively, antinociception is assessed as a statistically significant decrease in class 1 worms and an increase in the lower behavioral classes. When the worms were exposed to 10−5 M morphine (2.5 nmoles/mg), the percentage of individuals displaying a class 1 behavior dropped to 50% ± 5% (ANOVA P < 0.001; Figs. 1 and 2). Increasing concentrations of morphine, i.e., 10−4 M (25 pmol/mg) caused a greater antinociceptive effect, i.e., 37% ± 4% class 1 (Fig. 2). Exposure to concentrations of morphine lower than 10−5 M also decreased the antinociceptive effect but the differences with the control group were not statistically significant (Fig. 2). Morphine's antinociceptive effect on thermal avoidance was reversed by naloxone (77% ± 3%) and CTOP (76% ± 6%; Fig. 3). Similarly, the effect of 10−5 M EM1 and EM2 (2.5 nmoles/mg) on Tav was statistically significant reducing the percentage of worms of class I to 36% ± 4% and 39% ± 11%, respectively (Fig. 1; ANOVA P < 0.001). As with morphine, the antinociceptive effect of EM1 and EM2 was concentration dependent but the effect was insignificant for concentrations lower than 10−6 M (250 pmol/mg; Fig. 2). Worms treated with the opioid antagonists Naloxone and CTOP followed by treatment with either one of the agonists did not behave significantly different from the control worms (Fig. 3). The antinociceptive effect of morphine, EM1, and EM2 was reversed with the opioid receptor antagonists naloxone and CTOP (Fig. 3). The differences in percentage of worms in class 1 between the control group and the groups treated with both antagonists followed by the agonists were in all instances non-statistically significant. Nevertheless, CTOP by itself caused a significant drop in percentage of worms in class 1, 46% ± 2% (Fig. 3; ANOVA P < 0.05). Naloxone alone did not cause a significant change in percentage of class 1 behavior.
Fig. 1.
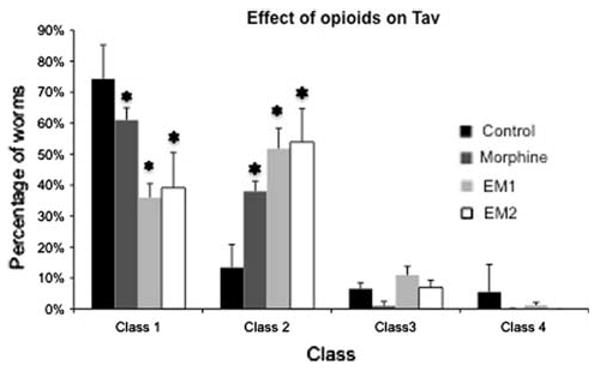
Thermal avoidance behavior of worms to noxious heat stimulus. The behavior of worms can be classified in four classes as shown above. Percentages shown for the control represent the baseline for Tav with 76% of the control worms belonging to class 1, 10% to class 2, 6% to class 3, and 7% to class 4. Worms treated with morphine, EM1, and EM2 showed a significant decrease in class 1 and proportional significant increases in class 2 (* P < 0.001) but not in classes 3 and 4 (ANOVA followed by Holm-Sidak method for multiple comparisons)
Fig. 2.
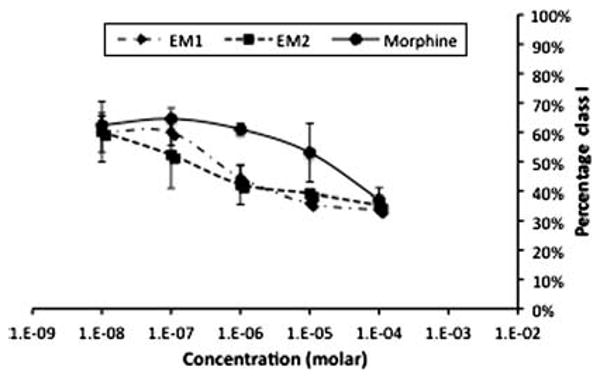
Dose–response curve for opioids effect on Tav. Worms were exposed to increasing concentrations of morphine, EM1, and EM2 ranging from 2.5 pmol/mg (10−8 M) to 25 nmol/mg (10−4 M). There is a positive correlation between drug concentration and percentage of worms belonging to class I. At concentrations higher than 0.25 nmol/mg of worm for all the drugs, the percentage of class 1 worms do not increase significantly
Fig. 3.
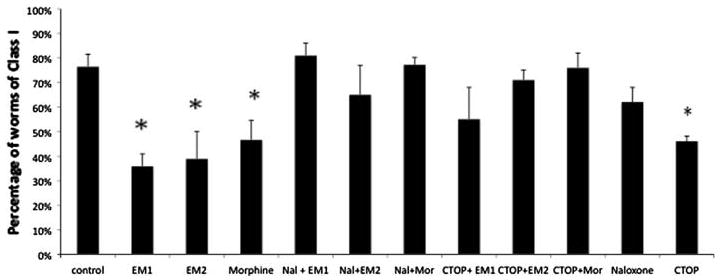
Effect of opioid agonists and their receptor antagonists on Tav. Morphine, EM1, and EM2 at a concentration of 10−5 M (2.5 nmoles/mg) caused a significant reduction in class 1 worm percentage. The generalist opioid receptor antagonist naloxone and the μ opioid receptor antagonist reversed the effect of the agonists. CTOP had a significant antinociceptive effect (P < 0.05)
Discussion
The effect of morphine on the behavioral response of invertebrates to aversive stimuli is documented in the literature (Achaval et al. 2005; Barr et al. 2008; Kalil-Gaspar et al. 2007; Kavaliers et al. 1983, 1998; Lozada et al. 1988; Maldonado et al. 1989; Pryor et al. 2007; Romano et al. 1990; Romero et al. 1994). In all instances, morphine increases the latency response of the animals and opioid receptor antagonists reverse this effect. This effect of morphine on thermonocifensive behavior has also been demonstrated in the parasitic nematode Ascaris suum (Pryor et al. 2007). This effect was also reversed by the opioid receptor antagonists naloxone and/or CTOP. Morphine-induced thermal antinociceptive responses have also been documented in the snail Cepeae nemoralis (Kavaliers et al. 1999). After 7–9 days of treatment with morphine at 10 mg/kg, the animal developed tolerance to morphine-induced analgesia (Kavaliers et al. 1999). A similar response has been shown in the cricket Pteronemobius sp for which 90 min after drug injections of 0.50 mg/g of morphine caused a 50% escape reaction time (ERT) increase (Zabala and Gomez 1991). The latency period of the biphasic avoidance response to noxious heat by the gastropod Megalobulimus abbreviatus increased when exposed to concentration of morphine, an effect reversed by naloxone (Achaval et al. 2005). All of the above support the existence of an opioid-mediated modulatory mechanism for nociception in invertebrates.
C. elegans exhibit thermal/warm avoidance as a survival behavior (Wittenburg and Baumeister 1999). Our results indicate that exogenous morphine, EM1, and EM2 affect an antinociceptive response to thermal avoidance. This response is concentration dependent and is reversed by naloxone and CTOP. These findings would support the existence of an opiate-like mechanism modulating the thermonocifensive response in C. elegans. Similar results had already been found previously in the parasitic nematode A. suum (Pryor et al. 2007). The precursor genes for this family and their receptors have not been described in C. elegans. It could be surmised that morphine may be acting through an opioid receptor subtype specific to C. elegans. Opioid receptors are G-Protein-coupled receptors (GPCR) of the Rhodopsin family. There are a total of 1,000 GPCRs in the genome of C. elegans of which 130 are putative neuropeptide receptors but only a few have a known cognate ligand (Keating et al. 2003). There are reports in the literature that support the view that the delta and mu opioid receptors subtypes evolved among the vertebrate taxa from an ancestor kappa-like subtype receptor that would have a single extracellular loop (EL) domain (Stevens 2004). The same researchers propose on the basis of phylogenetic analysis of gene sequences for the three opioid receptor subtypes that the receptor selectivity is greater in mammals than in any other vertebrate taxa since the degree of homology among sequences for the mu receptor is also greater among mammals (Stevens 2004). This gene family is absent of the genome of the ecdysozoans C. elegans and D. melanogaster (Dores et al. 2002). Nevertheless, this hypothesis discards a significant number of reports that support the presence of all the opioid receptor subtypes and their ligands among invertebrate taxa (Hanke et al. 1997; Kavaliers and Perrot-Sinal 1996; Kreienkamp et al. 2002; Liu et al. 1996; Makman 1994; Nieto-Fernandez et al. 1999; Salzet et al. 1997; Salzet and Stefano 1997a, b; Stefano et al. 1993, 1995; Stefano and Scharrer 1996; Zipser et al. 1988).
An alternate explanation for the phenomenon described herein is that morphine and the opioids EM1 and EM2 are acting through a FMRFamide-related peptide (FaRP) receptor. There is significant evidence of an interaction between the opioid system and the Phe-Met-Arg-Phe-NH2 (FMRF-amide) neuropeptide family (Tang et al. 1984; Yang et al. 1985). Tav is mediated at the nervous system level by the neurotransmitter glutamate and modulated in part by FMRFamide-related neuropeptides (FARPs; Wittenburg and Baumeister 1999). FMRFamide has been shown to induce a slight antinociceptive effect and also to antagonize opioid and morphine-induced analgesia (Kavaliers and Hirst 1985; Tang et al. 1984; Yang et al. 1985). It has been proposed that FMRFamide and its related FARPs neuropeptide family may be secreted after prolonged exposure to morphine but its actions are mediated by a separate set of specific receptors (Khan et al. 1998). This mechanism appears to be responsible in part for the development of tolerance to morphine. FARPs and opioid neuropeptides appear to be ancient neuropeptide families that are phylogenetically related. The neuropeptide precursor, Met-enkephalin-Arg-Phe, contains the FMRFamide sequence and it affects a naloxone reversible antinociception in mice (Gupta et al. 1999).
In summary, the results presented herein show that morphine, EM1, and EM2 affect an antinociceptive response in C. elegans as manifested by a significant decrease in the percentage of worms displaying a class I Tav response. This effect is reversed by Naloxone and CTOP supporting the presence of an opioid-mediated mechanism modulating thermonocifensive responses in C. elegans.
Acknowledgments
This work was supported by grants from the NIMH (COR 17138 and MRISP 47392) and the Research Foundation and Central Administration of the State University of New York.
References
- Achaval M, Penha MA, Swarowsky A, Rigon P, Xavier LL, Viola GG, Zancan DM. The terrestrial Gastropoda Megalobulimus abbreviatus as a useful model for nociceptive experiments: effects of morphine and naloxone on thermal avoidance behavior. Braz J Med Biol Res. 2005;38:73–80. doi: 10.1590/s0100-879x2005000100012. [DOI] [PubMed] [Google Scholar]
- Barr S, Laming PR, Dick JTA, Elwood RW. Nociception or pain in a decapod crustacean? Anim Behav. 2008;75:745–751. [Google Scholar]
- Cadet P, Stefano GB. Mytilus edulis pedal ganglia express mu opiate receptor transcripts exhibiting high sequence identity with human neuronal mu1. Brain Res Mol Brain Res. 1999;74:242–246. doi: 10.1016/s0169-328x(99)00287-9. [DOI] [PubMed] [Google Scholar]
- Cadet P, Zhu W, Mantione KJ, Baggerman G, Stefano GB. Cold stress alters Mytilus edulis pedal ganglia expression of mu opiate receptor transcripts determined by real-time RT-PCR and morphine levels. Brain Res Mol Brain Res. 2002;99:26–33. doi: 10.1016/s0169-328x(01)00342-4. [DOI] [PubMed] [Google Scholar]
- Dores RM, Lecaude S, Bauer D, Danielson PB. Analyzing the evolution of the opioid/orphanin gene family. Mass Spectrom Rev. 2002;21:220–243. doi: 10.1002/mas.10029. [DOI] [PubMed] [Google Scholar]
- Dureus P, Louis D, Grant AV, Bilfinger TV, Stefano GB. Neuropeptide Y inhibits human and invertebrate immunocyte chemotaxis, chemokinesis, and spontaneous activation. Cell Mol Neurobiol. 1993;13:541–546. doi: 10.1007/BF00711462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvaux-Miret D, Stefano GB, Smith EM, Mallozzi LA, Capron A. Proopiomelanocortin-derived peptides as tools of immune evasion for the human trematode schistosoma mansoni. Acta Biol Hung. 1992;43:281–286. [PubMed] [Google Scholar]
- Duvaux-Miret O, Leung MK, Capron A, Stefano GB. Schistosoma mansoni: an enkephalinergic system that may participate in internal and host-parasite signaling. Exp Parasitol. 1993;76:76–84. doi: 10.1006/expr.1993.1009. [DOI] [PubMed] [Google Scholar]
- Dyakonova VE. Role of opioid peptides in behavior of invertebrates. J Evol Biochem Physiol. 2001;37:335–347. [Google Scholar]
- Dyakonova VE, Schurmann F, Sakharov DA. Effects of serotonergic and opioidergic drugs on escape behaviors and social status of male crickets. Naturwissenschaften. 1999;86:435–437. doi: 10.1007/s001140050647. [DOI] [PubMed] [Google Scholar]
- Dyakonova V, Schormann FW, Sakharov DA. Social aggressiveness of female and subordinate male crickets is released by opiate receptor antagonist. Acta Biol Hung. 2000;51:363–367. [PubMed] [Google Scholar]
- Goumon Y, Casares F, Pryor S, Ferguson L, Brownawell B, Cadet P, Rialas CM, Welters ID, Sonetti D, Stefano GB. Ascaris suum, an intestinal parasite, produces morphine. J Immunol. 2000;165:339–343. doi: 10.4049/jimmunol.165.1.339. [DOI] [PubMed] [Google Scholar]
- Greenberg MJ, Painter SD, Doble KE, Nagle GT, Price DA, Lehman HK. The molluscan neurosecretory peptide FMRFamide: comparative pharmacology and relationship to the enkephalins. Fed Proc. 1983;42:82–86. [PubMed] [Google Scholar]
- Gupta S, Pasha S, Gupta YK, Bhardwaj DK. Chimeric peptide of Met-enkephalin and FMRFa induces antinociception and attenuates development of tolerance to morphine antinociception. Peptides. 1999;20:471–478. doi: 10.1016/s0196-9781(99)00028-5. [DOI] [PubMed] [Google Scholar]
- Hanke J, Willig A, Yinon U, Jaros PP. Delta and kappa opioid receptors in eyestalk ganglia of a crustacean. Brain Res. 1997;744:279–284. doi: 10.1016/S0006-8993(96)01114-6. [DOI] [PubMed] [Google Scholar]
- Harrison LM, Kastin AJ, Weber JT, Banks WA, Hurley DL, Zadina JE. The opiate system in invertebrates. Peptides. 1994;15:1309–1329. doi: 10.1016/0196-9781(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kalil-Gaspar P, Marcuzzo S, Rigon P, Molina CG, Achaval M. Capsaicin-induced avoidance behavior in the terrestrial Gastropoda Megalobulimus abbreviatus: evidence for TRPV-1 signaling and opioid modulation in response to chemical noxious stimuli. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:286–291. doi: 10.1016/j.cbpa.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Hirst M. FMRFamide, a putative endogenous opiate antagonist: evidence from suppression of defeat-induced analgesia and feeding in mice. Neuropeptides. 1985;6:485–494. doi: 10.1016/0143-4179(85)90110-6. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Perrot-Sinal TS. Pronociceptive effects of the neuropeptide, nociceptin, in the land snail, Cepaea nemoralis. Peptides. 1996;17:763–768. doi: 10.1016/0196-9781(96)00105-2. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Hirst M, Teskey GC. A functional role for an opiate system in snail thermal behavior. Science. 1983;220:99–101. doi: 10.1126/science.6298941. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Prato FS, Ossenkopp KP. Evidence for the involvement of nitric oxide and nitric oxide synthase in the modulation of opioid-induced antinociception and the inhibitory effects of exposure to 60-Hz magnetic fields in the land snail. Brain Res. 1998;809:50–57. doi: 10.1016/s0006-8993(98)00844-0. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD, Choleris E. Parasites and behavior: an ethopharmacological analysis and biomedical implications. Neurosci Biobehav Rev. 1999;23:1037–1045. doi: 10.1016/s0149-7634(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Keating CD, Kriek N, Daniels M, Ashcroft NR, Hopper NA, Siney EJ, Holden-Dye L, Burke JF. Whole-genome analysis of 60 G protein-coupled receptors in Caenorhabditis elegans by gene knockout with RNAi. Curr Biol. 2003;13:1715–1720. doi: 10.1016/j.cub.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Khan FA, Jain MR, Saha SG, Subhedar N. FMRFamide-like immunoreactivity in the olfactory system responds to morphine treatment in the teleost Clarias batrachus: involvement of opiate receptors. Gen Comp Endocrinol. 1998;110:79–87. doi: 10.1006/gcen.1997.7044. [DOI] [PubMed] [Google Scholar]
- Kreienkamp HJ, Larusson HJ, Witte I, Roeder T, Birgul N, Honck HH, Harder S, Ellinghausen G, Buck F, Richter D. Functional annotation of two orphan G-protein-coupled receptors, Drostar1 and -2, from Drosophila melanogaster and their ligands by reverse pharmacology. J Biol Chem. 2002;277:39937–39943. doi: 10.1074/jbc.M206931200. [DOI] [PubMed] [Google Scholar]
- Leung MK, Dissous C, Capron A, Woldegaber H, Duvaux-Miret O, Pryor S, Stefano GB. Schistosoma mansoni: the presence and potential use of opiate-like substances. Exp Parasitol. 1995;81:208–215. doi: 10.1006/expr.1995.1110. [DOI] [PubMed] [Google Scholar]
- Li C, Nelson LS, Kim K, Nathoo A, Hart AC. Neuropeptide gene families in the nematode Caenorhabditis elegans. Ann N Y Acad Sci. 1999;897:239–252. doi: 10.1111/j.1749-6632.1999.tb07895.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shenouda D, Bilfinger TV, Stefano ML, Magazine HI, Stefano GB. Morphine stimulates nitric oxide release from invertebrate microglia. Brain Res. 1996;722:125–131. doi: 10.1016/0006-8993(96)00204-1. [DOI] [PubMed] [Google Scholar]
- Lozada M, Romano A, Maldonado H. Effect of morphine and naloxone on a defensive response of the crab Chasmagnathus granulatus. Pharmacol Biochem Behav. 1988;30:635–640. doi: 10.1016/0091-3057(88)90076-7. [DOI] [PubMed] [Google Scholar]
- Makman MH. Morphine receptors in immunocytes and neurons. Adv Neuroimmunol. 1994;4:69–82. doi: 10.1016/s0960-5428(05)80002-6. [DOI] [PubMed] [Google Scholar]
- Maldonado H, Romano A, Lozada M. Opioid action on response level to a danger stimulus in the crab (Chasmagnathus granulatus) Behav Neurosci. 1989;103:1139–1143. doi: 10.1037//0735-7044.103.5.1139. [DOI] [PubMed] [Google Scholar]
- Nieto-Fernandez FE, Mattocks D, Cavani F, Salzet M, Stefano GB. Morphine coupling to invertebrate immunocyte nitric oxide release is dependent on intracellular calcium transients. Comp Biochem Physiol B Biochem Mol Biol. 1999;123:295–299. doi: 10.1016/s0305-0491(99)00074-7. [DOI] [PubMed] [Google Scholar]
- Pryor SC, Elizee R. Evidence of opiates and opioid neuropeptides and their immune effects in parasitic invertebrates representing three different phyla: schistosoma mansoni, Theromyzon tessulatum, Trichinella spiralis. Acta Biol Hung. 2000;51:331–341. [PubMed] [Google Scholar]
- Pryor SC, Nieto F, Henry S, Sarfo J. The effect of opiates and opiate antagonists on heat latency response in the parasitic nematode Ascaris suum. Life Sci. 2007;80:1650–1655. doi: 10.1016/j.lfs.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Renaud FL, Colon I, Lebron J, Ortiz N, Rodriguez F, Cadilla C. A novel opioid mechanism seems to modulate phagocytosis in Tetrahymena. J Eukaryot Microbiol. 1995;42:205–207. doi: 10.1111/j.1550-7408.1995.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Renaud FL, Chiesa R, Rodriguez F, Tomassini N, Marino M. Studies on the opioid mechanism in Tetrahymena. Prog Mol Subcell Biol. 1996;17:29–39. doi: 10.1007/978-3-642-80106-8_2. [DOI] [PubMed] [Google Scholar]
- Renzelli-Cain R, Kaloustian KV. Evidence for the involvement of opioid peptides in phagocytosis, conformation, granulation and aggregation of immunocompetent Lumbricus terrestris amoebocytes. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1995;111:205–211. doi: 10.1016/0742-8413(95)00036-n. [DOI] [PubMed] [Google Scholar]
- Romano A, Lozada M, Maldonado H. Effect of naloxone pretreatment on habituation in the crab chasmagnathus granulatus. Behav Neural Biol. 1990;53:113–122. doi: 10.1016/0163-1047(90)90882-7. [DOI] [PubMed] [Google Scholar]
- Romero SMB, Hoffmann A, Menescal-de-Oliveira L. Is there an opiate receptor in the snail Megalobulimus sanctipauli? Action of morphine and naloxone. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1994;107:37–40. [Google Scholar]
- Ryu WS, Samuel ADT. Thermotaxis in Caenorhabditis elegans analyzed by measuring responses to defined thermal stimuli. J Neurosci. 2002;22:5727–5733. doi: 10.1523/JNEUROSCI.22-13-05727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzet M. Neuroimmunology of opioids from invertebrates to human. Neuro Endocrinol Lett. 2001;22:467–474. [PubMed] [Google Scholar]
- Salzet M, Stefano G. Prodynorphin in invertebrates. Mol Brain Res. 1997a;52:46–52. doi: 10.1016/s0169-328x(97)00200-3. [DOI] [PubMed] [Google Scholar]
- Salzet M, Stefano GB. Invertebrate proenkephalin: delta opioid binding sites in leech ganglia and immunocytes. Brain Res. 1997b;768:224–232. doi: 10.1016/s0006-8993(97)00646-x. [DOI] [PubMed] [Google Scholar]
- Salzet M, Salzet-Raveillon B, Cocquerelle C, Verger-Bocquet M, Pryor SC, Rialas CM, Laurent V, Stefano GB. Leech immunocytes contain proopiomelanocortin: nitric oxide mediates hemolymph proopiomelanocortin processing. J Immunol. 1997;159:5400–5411. [PubMed] [Google Scholar]
- Sonetti D, Ottaviani E, Stefano GB. Opiate signaling regulates microglia activities in the invertebrate nervous system. Gen Pharmacol. 1997;29:39–47. doi: 10.1016/s0306-3623(96)00523-x. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Scharrer B. The presence of the mu3 opiate receptor in invertebrate neural tissues. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;113:369–373. doi: 10.1016/0742-8413(96)02111-1. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Digenis A, Spector S, Leung MK, Bilfinger TV, Makman MH, Scharrer B, Abumrad NN. Opiate-like substances in an invertebrate, an opiate receptor on invertebrate and human immunocytes, and a role in immunosuppression. Proc Natl Acad Sci USA. 1993;90:11099–11103. doi: 10.1073/pnas.90.23.11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano GB, Casares F, Liu U. Naltrindole sensitive delta 2 opioid receptor mediates invertebrate immunocyte activation. Acta Biol Hung. 1995;46:321–327. [PubMed] [Google Scholar]
- Stefano GB, Cadet P, Rialas CM, Mantione K, Casares F, Goumon Y, Zhu W. Invertebrate opiate immune and neural signaling. Adv Exp Med Biol. 2003;521:126–147. [PubMed] [Google Scholar]
- Stevens CW. Opioid research in amphibians: an alternative pain model yielding insights on the evolution of opioid receptors. Brain Res Rev. 2004;46:204–215. doi: 10.1016/j.brainresrev.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Yang HY, Costa E. Inhibition of spontaneous and opiate-modified nociception by an endogenous neuropeptide with Phe-Met-Arg-Phe-NH2-like immunoreactivity. Proc Natl Acad Sci USA. 1984;81:5002–5005. doi: 10.1073/pnas.81.15.5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenburg N, Baumeister R. Thermal avoidance in Caenorhabditis elegans: an approach to the study of nociception. Proc Natl Acad Sci USA. 1999;96:10477–10482. doi: 10.1073/pnas.96.18.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Fratta W, Majane EA, Costa E. Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc Natl Acad Sci USA. 1985;82:7757–7761. doi: 10.1073/pnas.82.22.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabala NA, Gomez MA. Morphine analgesia, tolerance and addiction in the cricket Pteronemobius sp. (Orthoptera, Insecta) Pharmacol Biochem Behav. 1991;40:887–891. doi: 10.1016/0091-3057(91)90102-8. [DOI] [PubMed] [Google Scholar]
- Zhu W, Pryor SC, Putnam J, Cadet P, Stefano GB. Opiate alkaloids and nitric oxide production in the nematode Ascaris suum. J Parasitol. 2004;90:15–22. doi: 10.1645/GE-3208. [DOI] [PubMed] [Google Scholar]
- Zipser B, Ruff MR, O'Neill JB, Smith CC, Higgins WJ, Pert CB. The opiate receptor: a single 110 kDa recognition molecule appears to be conserved in Tetrahymena, leech, and rat. Brain Res. 1988;463:296–304. doi: 10.1016/0006-8993(88)90403-9. [DOI] [PubMed] [Google Scholar]


