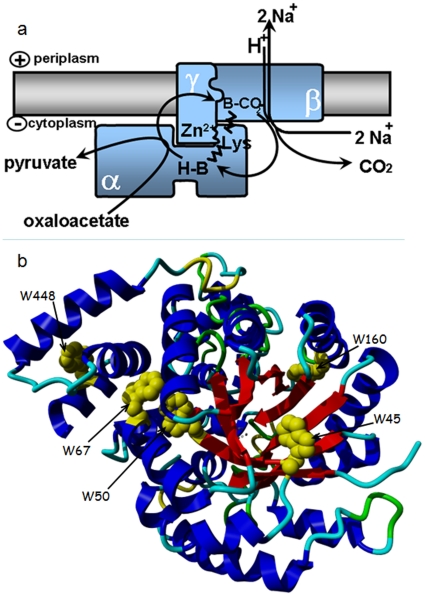
Figure 1. Organization of the OAD complex.
a. Structural model and catalytic events of the oxaloacetate decarboxylase. Oxaloacetate decarboxylase is a membrane-bound enzyme complex composed of α, β, and γ subunits in a 1∶1∶1 molar ratio. The α subunit is soluble and harbors the carboxyltransferase catalytic site. The carboxyl group from position 4 of oxaloacetate is transferred to the biotin prosthetic group bound to the C-terminal biotin-binding domain. The carboxybiotin formed switches to the decarboxylase site on subunit β, where decarboxylation takes place and free biotin is regenerated, using one periplasmic proton. During the reaction, two sodium ions are translocated from the cytoplasm into the periplasm. Adapted from [9]. b. Structure of the carboxyltransferase domain of OAD α subunit highlighting the position of tryptophan residues. Four of them (positions 45, 50, 67, 160) are located within the catalytic α8β8 subdomain whereas a fifth tryptophan (W448) is located in the noncatalytic subdomain. This figure was drawn using YASARA (www.yasara.org) from PDB file 2NX9.