Abstract
Purpose
The surgical work force distribution at the county level varies widely across the United States, and the impact of differential access on cancer outcomes is unclear. We used urologists as a test case because they are the first care providers for urologic cancers, can easily be identified from available data sources, and are unevenly distributed throughout the country. The goal of this study was to determine the effect of increasing urologist density on local prostate, bladder, and kidney cancer mortality.
Patients and Methods
Using county-level data from the Area Resource File, US Census, National Cancer Institute, and Centers for Disease Control, regression models were built for prostate, bladder, and kidney cancer mortality, controlling for categorized urologist density, county demographics, socioeconomic factors, and preexisting health care infrastructure.
Results
For each of the three cancers, there was a statistically significant cancer-specific mortality reduction associated with counties that had more than zero urologists (16% to 22% reduction for prostate cancer, 17% to 20% reduction for bladder cancer, and 8% to 14% reduction for kidney cancer with increasing urologist density) relative to zero urologists. However, increasing density greater than two urologists per 100,000 people had no statistically significant impact on mortality for any of the tumors studied.
Conclusion
The presence of a urologist is associated with lower mortality for urologic cancers in that county, but increasing urologist density does not yield further improvements. Therefore, a nuanced and geographically aware policy toward the size and distribution of the future work force is most likely to provide the greatest population-level improvement in cancer mortality outcomes.
INTRODUCTION
As the US health system faces significant financial and demographic pressures, the composition, efficiency, and adequacy of the physician work force has come under closer scrutiny. The proportion of the US population older than 65 years is projected to grow from 13% to 19% by 2030,1 and some argue that the graying of the American population will lead to a dramatic shortage of generalists and specialists,2,3 whereas others believe that specifically increasing the primary care work force and expanding access will be the most efficient means of improving population health outcomes.4–6
In a meta-analysis of 10 studies assessing the impact of increased primary care density, Macinko et al4 concluded that adding one additional primary care MD (PMD) per 10,000 people was associated with 5.3% improvement in all-cause mortality. PMD density has also been associated with lower rates of cancer mortality, earlier breast and colon cancer detection, and lower heart disease mortality.7–9 However, an analysis using geographically weighted regression, which adjusts data based on the physical distance separating the geographic regions from which the data are drawn, found marked regional variation, which may obscure true trends when analyzed at a national level.10 Some have found specialists to be associated with improved disease-specific outcomes, such as improved melanoma mortality outcomes in areas served by dermatologists and lower neonatal mortality in areas that have some neonatologists (more than the lowest quintile).11,12
Using travel time to the nearest cancer facility to approximate access to care, overall cancer care availability has been shown to be widely variable throughout the United States.13 However, little data exist addressing population-based cancer outcomes in relationship to the disparity in access, particularly the available cancer clinician work force. The heterogeneous nature of cancer and the multidisciplinary approaches often used further complicate work force and outcomes analysis.
In response to these limitations of the extant literature, this analysis focuses on the urologist work force because urologists often both diagnose and provide primary surgical and medical oncologic care for prostate, bladder, and kidney cancer. The goal of this study was to determine the effect of increasing urologist density on local prostate, bladder, and kidney cancer mortality for an understanding of the effect of increasing clinician density on cancer mortality outcomes.
PATIENTS AND METHODS
This investigation used an ecologic study design with the county, as defined by the 2000 US Census, as the geographic unit of analysis. Of 3,141 counties in the United States, we excluded all rural counties (669, or 21.3%) because only 4% of rural counties had any urologists, and mortality data were reported for less than 2.5% of these counties.
Data Sources
Prostate, bladder, and kidney cancer incidence data at the county level were obtained from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program and the National Program of Cancer Registries (NPCR), and mortality data were obtained from the US Centers for Disease Control and Prevention's National Vital Statistics System and NPCR. These data have been merged and published online.14 Incidence is reported as age-adjusted average annual incidence (2001 to 2005 for SEER regions, 2001 to 2004 for non-SEER regions), and mortality is reported as age-adjusted average annual death rate (2001 to 2005). Both incidence and mortality are reported as events per 100,000 people and are assigned to counties based on each individual's residential address at the time of diagnosis (per SEER) and death (per death certificates via NPCR). Of 2,472 nonrural counties in the United States, 1,012 counties (41%) had complete incidence and mortality data for prostate cancer, 571 counties (23%) had complete data for bladder cancer, and 595 counties (24%) had complete data for kidney cancer.
Demographic data and physician distribution were obtained from the Area Resource File 200615 (ARF) published by the Health Resources and Services Administration of the US Department of Health and Human Services. The ARF aggregates and reports data from more than 50 sources, including the US Census, Bureau of Economic Analysis, Bureau of Labor Statistics, and others. The ARF includes the number of physicians by specialty per county, based on the American Medical Association Physician Masterfile. Only those who have completed residency training are included in this analysis; PMDs include only those with training in general practice, family practice, or general internal medicine. All physician totals used are mean MDs per 100,000 people standardized to mean annual US Census county population estimates (2001 to 2005).
Counties were classified as metropolitan, nonmetropolitan, and rural, based on Department of Agriculture 2003 Rural/Urban Continuum Codes16 and as 2004 Primary Care Health Professional Shortage Areas (HPSA) in the ARF. The ARF was used to collect data on average hospital beds per 100,000 people, unemployment rates, and median per capita income (2001 to 2004). Additional demographic data used included Census 2000 levels of educational attainment and Census 2001 to 2004 estimates of ethnic distribution and age. The proportion with health insurance in each county was obtained from the US Census Small Area Health Insurance Estimates 2000.17
Statistical Analyses
The primary outcome measure was cancer mortality per 100,000 people at the county level. Due to a highly skewed urologist distribution, with 49% of nonrural counties not having a urologist between 2001 and 2005, urologist density was categorized (0, 0.1 to 2.0, 2.1 to 4.0, 4.1 to 6.0, and ≥ 6.1 urologists per 100,000 people). All statistical analyses were performed separately for each of the three cancers of interest: prostate, bladder, and kidney. Univariate associations between each of the predictor variables (urologist density, incidence, county metropolitan status, primary care density, HPSA status, hospital bed density, median age in county, median income, unemployment rate, percentage minority population, education, and health insurance coverage) and cancer mortality were tested using t test for categoric predictors and simple linear regression for continuous predictors. Independent multivariate regression models were built for each cancer using backwards stepwise selection with a univariate P < .15 for initial inclusion into the model and P < .05 as a final inclusion cutoff. The baseline comparator in all analyses is a nonmetropolitan county with zero urologists but was not classified as a primary care HPSA. Variance inflation factors, a measure of collinearity and an indicator of inaccurate estimates, were used to assess for interaction between variables. Statistical analysis was performed using Stata (version 10; Stata, College Station, TX).
RESULTS
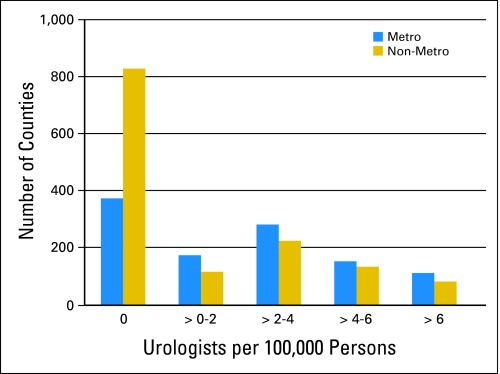
Overall prostate, bladder, and kidney cancer incidence and mortality rates are reported for metropolitan and nonmetropolitan counties with available data (Table 1). There were no urologists present in 34% of metropolitan counties and 60% of nonmetropolitan counties, reflecting an uneven distribution consistent with prior work (Fig 1). In addition, metropolitan counties had a lower rate of cancer-specific mortality independent of urologist density (prostate, 8.1% lower; 95% CI, 5.2% to 10.9%; bladder, 9.1% lower, 95% CI, 5.6% to 12.6%; kidney, 12.8% lower, 95% CI, 9.4% to 16.1%).
Table 1.
Incidence (age-adjusted average annual incidence) and Mortality (age-adjusted average annual deaths) for Metropolitan and Nonmetropolitan Counties, 2001 Through 2005
| Type of Cancer | Incidence |
Mortality |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Prostate cancer | 149.2 | 34.3 | 29.7 | 8.6 |
| Bladder cancer | 22.1 | 5.3 | 4.8 | 1.2 |
| Kidney cancer | 14.7 | 3.2 | 4.7 | 1.2 |
NOTE. Reported as events per 100,000 people in county. Data are from the National Cancer Institute's Surveillance, Epidemiology, and End Results program and the US Centers for Disease Control and Prevention's National Vital Statistics System and National Program of Cancer Registries.
Abbreviation: SD, standard deviation.
Fig 1.
Distribution of urologist density in metropolitan and nonmetropolitan counties.
Prostate Cancer
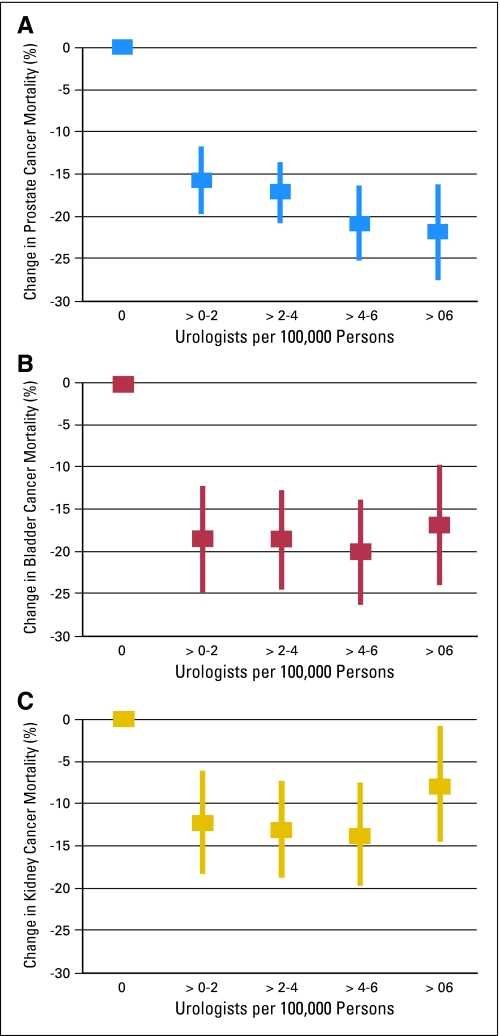
The greatest change in prostate cancer mortality comes with the addition of the first urologist in a county, being associated with at least 15.7% lower mortality rate (95% CI, 11.7% to 19.7%). Although the mortality reduction increases further with increasing urologist density, these improvements are not statistically significant. The mortality reduction from 0.1 to 2.0 to 2.1 to 4.0 urologists per 100,000 people is an additional 1.5% (odds ratio [OR] = 0.61; 95% CI, 0.17 to 2.20), from 2.1 to 4.0 to 4.1 to 6.0 is 3.6% (OR = 0.30; 95% CI, 0.09 to 1.01), and from 4.1 to 6.0 to ≥ 6.1 is 1.1% (OR = 0.69, 95% CI, 0.13 to 3.77).
The baseline annual mortality rate was 34.1 per 100,000 people and associated with 8% lower mortality in metropolitan counties (Table 2). Counties classified as metropolitan were associated with 8% lower prostate cancer mortality than nonmetropolitan counties in our multivariate model, which was consistent with both univariate analyses and bladder and kidney cancer results. The availability of primary care services had a large impact on prostate cancer mortality: counties that were complete primary care HPSAs we associated with a 10.3% increase in prostate cancer mortality, although the incremental effect of each additional PMD per 100,000 was minimal.
Table 2.
Predictors of Prostate Cancer Mortality: Multivariate Regression With Centered Variables
| Variable | % Change in Prostate Cancer Mortality | P | 95% CI |
|---|---|---|---|
| Urologists per 100,000 people | |||
| 0.1-2.0 v 0 | −15.73 | .000 | −19.72 to −11.74 |
| 2.1-4.0 v 0 | −17.20 | .000 | −20.80 to −13.60 |
| 4.1-6.0 v 0 | −20.77 | .000 | −25.20 to −16.35 |
| ≥ 6.1 v 0 | −21.87 | .000 | −27.52 to −16.21 |
| Incidence: Each percent increase | 0.16 | .000 | 0.10 to 0.23 |
| Metropolitan: If county is metropolitan, compared with nonmetropolitan | −8.06 | .000 | −10.94 to −5.18 |
| Primary care: Each additional primary care MD per 100,000 people | 0.07 | .001 | 0.03 to 0.11 |
| Primary care: If county is an HPSA | 10.26 | .000 | 4.87 to 15.65 |
| Facilities: Each 1% increase in hospital beds per 100,000 people | 3.05 | .005 | 0.92 to 5.17 |
| Age: Each percentage point increase in percentage of population older than 65 years | −0.95 | .000 | −1.32 to −0.57 |
| Income: Each $1k increase in median per capita income | −0.38 | .004 | −0.64 to −0.12 |
| Ethnicity: Each 1% percent increase in minority population | 5.76 | .000 | 4.30 to 7.21 |
| Education: Each percentage point increase in percent of population with high school diploma | −0.47 | .000 | −0.71 to −0.23 |
| Insurance: Each percentage point increase in percent of population without health insurance | −0.65 | .001 | −1.05 to −0.26 |
| Reference group: Prostate cancer mortality per 100,000 people in nonmetropolitan, non-HPSA county with 0 urologists | 34.077 | .000 | 33.045 to 35.109 |
NOTE. Unemployment rate was dropped from the analysis because it did not meet statistical significance inclusion criteria.
Abbreviation: HPSA, Health Professional Shortage Area.
Ethnicity was also strongly associated with prostate cancer mortality, with each percentage point increase in the proportion of non-Caucasians in a county associated with a 5.8% increase in prostate cancer mortality. This statistically significant increase was present despite controlling for income, insurance status, employment rates, and educational attainment and was not found for bladder or kidney cancers.
Bladder Cancer
The presence of more than 0 to 2 urologists per 100,000 people in the county was associated with 18% lower bladder cancer mortality in that county (95% CI, 12.1% to 24.6%). Mortality changes with additional urologists were not statistically significant: from 0.1 to 2.0 to 2.1 to 4.0 urologists per 100,000 people, mortality was 0.1% less (OR = 0.99; 95% CI, 0.78 to 1.26), from 2.1 to 4.0 to 4.1 to 6.0 was 1.5% less (OR = 0.91; 95% CI, 0.73 to 1.14), and from 4.1 to 6.0 to ≥ 6.1 mortality was 3.2% more (OR = 1.22; 95% CI, 0.89 to 1.68). The baseline annual mortality rate was 6.3 per 100,000 people and associated with 9% lower mortality in metropolitan counties (Table 3). For bladder cancer, mortality did not seem to be affected by primary care availability or preexisting health care infrastructure such as HPSA status, PMD density, and hospital bed density.
Table 3.
Predictors of Bladder Cancer Mortality: Multivariate Regression With Centered Variables
| Variable | % Change in Bladder Cancer Mortality | P | 95% CI |
|---|---|---|---|
| Urologists per 100,000 people | |||
| 0.1-2.0 v 0 | −18.33 | .000 | −24.59 to −12.08 |
| 2.1-4.0 v 0 urologists | −18.43 | .000 | −24.30 to −12.57 |
| 4.1-6.0 v 0 urologists | −19.89 | .000 | −26.08 to −13.69 |
| ≥ 6.1 v 0 urologists | −16.67 | .000 | −23.77 to −9.58 |
| Incidence: Each percent increase | 0.33 | .000 | 0.26 to 0.41 |
| Metropolitan: If county is metropolitan, compared with nonmetropolitan | −9.12 | .000 | −12.61 to −5.64 |
| Age: Each percentage point increase in percentage of population older than 65 years | −0.40 | .041 | −0.79 to −0.02 |
| Income: Each $1k increase in median per capita income | −0.28 | .012 | −0.50 to −0.06 |
| Insurance: Each percentage point increase in percent of population without health insurance | −0.45 | .024 | −0.84 to −0.06 |
| Reference group: Bladder cancer mortality per 100,000 people in nonmetropolitan county with 0 urologists | 6.282 | .000 | 5.924 to 6.639 |
NOTE. The following variables were dropped from the analysis because they did not meet statistical significance inclusion criteria: Primary care MD density, Health Professional Shortage Area status, hospital bed density, ethnic distribution, unemployment rate, and level of education in county.
Kidney Cancer
Just as with prostate and bladder cancer, the presence of urologists was associated with improved kidney cancer outcomes, as counties with 0.1 to 2.0 urologists per 100,000 had a 12.3% lower predicted mortality than those without any urologists (95% CI, 6.2% to 18.4%). The mortality reduction from 0.1 to 2.0 to 2.1 to 4.0 urologists per 100,000 people was an additional 0.8% (OR = 0.95; 95% CI, 0.76 to 1.18), from 2.1 to 4.0 to 4.1 to 6.0 is 0.6% (OR = 0.97; 95% CI, 0.78 to 1.19), and from 4.1 to 6.0 to ≥ 6.1 was worsened by 6.1% (OR = 1.45; 95% CI, 1.07 to 1.96). The baseline annual mortality rate was 6.2 per 100,000 people and associated 13% lower mortality in metropolitan counties (Table 4). The level of urbanization in a county did have a significant effect; metropolitan counties were associated with 13% lower kidney cancer mortality than comparable nonmetropolitan counties. Primary care availability and hospital bed density did not affect kidney cancer mortality in our models.
Table 4.
Predictors of Kidney Cancer Mortality: Multivariate Regression With Centered Variables
| Variable | % Change in Kidney Cancer Mortality | P | 95% CI |
|---|---|---|---|
| Urologists per 100,000 people | |||
| 0.1-2.0 v 0 | −12.33 | .000 | −18.43 to −6.23 |
| 2.1-4.0 v 0 | −13.16 | .000 | −18.93 to −7.39 |
| 4.1-6.0 v 0 | −13.73 | .000 | −19.85 to −7.62 |
| ≥ 6.1 v 0 | −7.68 | .032 | −14.68 to −0.67 |
| Incidence: Each percent increase | 0.45 | .000 | 0.38 to 0.53 |
| Metropolitan: If county is metropolitan, compared with nonmetropolitan | −12.77 | .000 | −16.13 to −9.42 |
| Age: Each percentage point increase in percentage of population older than 65 years | −0.79 | .000 | −1.16 to −0.42 |
| Income: Each $1k increase in median per capita income | −0.73 | .000 | −0.95 to −0.52 |
| Insurance: Each percentage point increase in percent of population without health insurance | −0.41 | .017 | −0.74 to −0.07 |
| Reference group: Kidney cancer mortality per 100,000 people in nonmetropolitan county with 0 urologists | 6.159 | .000 | 5.811 to 6.508 |
NOTE. The following variables were dropped from the analysis because they did not meet statistical significance inclusion criteria: Primary care MD density, Health Professional Shortage Area status, hospital bed density, ethnic distribution, unemployment rate, and level of education in county.
DISCUSSION
To properly assess the adequacy of the cancer clinician work force, physician density and access must be correlated with patient-centered outcomes. Onega et al13 have described access to cancer care using distance from a National Cancer Center Cancer Center as proxy measure. However, the de facto adequacy of access can only be determined by correlation with objective cancer-specific outcomes. The heterogeneity of cancer natural history and treatment modalities, which require differing levels of treatment intensity, necessitates more granular analysis of providers and the cancers they treat.
We previously noted that as of 2004, there were on average 1.6 urologists per 100,000 people in the United States and that urologist density is starkly uneven, with distribution favoring large urban centers and counties with high levels of education and mild climates.18 Looking at overall density, in 1978, Allen et al19 recommended one urologist per 35,000 people by 2020 (2.9 per 100,000). Ansell20 calculated national urologist density at one per 28,290 people (3.53 per 100,000) in 1983 and, on the basis of demographics, hospital bed density, and an informal survey of how busy urologists felt they were, concluded that was an adequate density. More recently, a 1995 report to the American Urological Association Executive Committee recommended one urologist per 50,000 people (two per 100,000).21 The only study to base its recommendations on specific disease- or outcomes-related data combined benign prostatic hypertrophy and incontinence incidence with demographic data and approximated the need for an additional 200 urologists per year.22
Like other work assessing the physician work force,2 these studies have used the historical and current supply as a foundation for future projections, and none have measured supply against objective patient or population-based outcomes, nor have any assessed the important geographic variation in clinician supply. Studies assessing the geographic variation in prostate cancer mortality have found clusters of high mortality unexplained by demographic or socioeconomic factors and an inverse relationship between local screening intensity and incidence of late-stage disease.23,24
The county-level supply of urologists seems to have a significant impact on prostate, bladder, and kidney cancer outcomes independent of demographics, socioeconomics, and preexisting health care infrastructure. We found that among all three cancers, counties with 0.1 to 2.0 urologists per 100,000 people had 12% to 18% lower mortality than those without any urologists. However, increased urologist density beyond this level yielded minimal additional improvements in cancer mortality, suggesting a rapid plateau effect (Fig 2). The mortality benefits did not improve in a statistically significant manner as density increased above two urologists per 100,000 people for any of the tumors analyzed. The increased mortality as urologist density surpasses six per 100,000 for kidney cancer is likely a statistical aberration due to the small number of counties in this category with available kidney cancer data and is supported by the wide CIs (0.7% to 14.7%).
Fig 2.
Change in cancer mortality with increasing urologist density in a county using data from multivariate models. (A) prostate; (B) bladder; (C) kidney.
We found that only prostate cancer mortality was sensitive to primary care availability as measured by primary care HPSAs. This may be due to more prostate cancer diagnoses resulting from PMD-initiated screening, whereas bladder and kidney cancer are often detected during evaluations for hematuria or as incidental radiographic findings. For prostate cancer, the minimal incremental increase in prostate cancer mortality with each additional PMD per 100,000 may suggest increased diagnosis and thus attribution of mortality to a cancer-specific cause and not a true increase in incidence. It also may suggest the need for some basal level of primary care access as measured by HPSA, but limited mortality benefit with increased PMD density.
When assessing multivariate predictors of cancer mortality at the county level, certain factors had a consistent effect among all three cancers (Tables 2 to 4). Each percentage increase in specific cancer incidence above the national mean was associated with a statistically significant 0.16% to 0.45% increase in mortality. In addition, metropolitan counties had 8% to 13% lower cancer mortality than comparable nonmetropolitan counties, independent of urologist density and all other covariates. For all three cancers, counties with higher median income had lower mortality rates. An increase in the proportion of a county's ethnic/racial minority population was independently associated with a statistically significant increase in prostate cancer mortality, but this trend was not found for bladder or kidney cancers.
Studies in other health care fields have found a similar plateau effect with increasing physician supply. Krakauer et al25 found that although doubling the physician density in the lowest decile is associated with lower ambulatory care sensitive admission rates (from approximately eight per 100 Medicare enrollees to five per 100), any increases above the first decile had no effect on admission rates, and that all-cause mortality was not influenced by physician supply. Similarly, Goodman12 reported a decrease in neonatal mortality above the lowest quintile of neonatologist supply, but no additional improvements with increasing neonatologist density.
Our data suggest counties need some access to urologic care for the largest improvements in urologic cancer mortality, but those that already have a urologist presence are unlikely to see improvements in cancer mortality with increased urologist density. These findings have important policy implications when put in the context of prior work from these data sets showing a potentially adequate national average (3.4 per 100,000), but a strikingly uneven distribution of urologists throughout the United States.18 Because work force policies may be insufficient to overcome the increasingly strong preference among younger physicians to cluster around urban areas,18 and a completely even distribution may not affect noncancer mortality outcomes, we must find other creative solutions for expanding access to urologic oncology care, such as tele-medicine or remote consultation.
Better understanding of regional clustering of incidence, mortality, and health care infrastructure is vital to developing effective work force and health delivery policies. As shown by the important role of clustering and regional variation in the relationship between physician supply and mortality geographic clustering,10 analyses using states as the unit of measure are likely to obscure important trends.26 Future work should use spatially aware statistical modeling techniques such as geographic weighted regression, which weighs the influence of each geographic unit's data based on its physical distance from other geographic units. In addition, future work should explore the relationship between physician density, utilization, and more nuanced outcome measures encompassing health-related quality of life.
Several caveats to this study should be noted. This study was designed as an ecologic study at the county level, and as a result, the conclusions cannot be applied to any unit smaller than the county, and inference about causality of the observed association must be made with caution. As with any study, accuracy of the results is dependent on the quality and completeness of input data. Although our data represent a majority of the population of the United States, incidence and mortality are not available for every county, and all rural counties were excluded due to limited data. Furthermore, cancer mortality is a crude outcome measure and is based on death certificate data, which are not perfectly accurate. The incidence data used do not account for stage at presentation and aggressiveness of disease. Further, our analysis does not account for morbidity or health-related quality of life related to either the disease or the quality of care because, to our knowledge, sufficiently granular data do not exist nationwide. We controlled for the quality of preexisting health infrastructure in a county with a combination of PMD density, hospital bed density, and health insurance coverage rates. We also controlled for socioeconomic variation by evaluating median income, unemployment rates, education levels, and ethnic distribution in each county. Because there is no perfect measure of overall health infrastructure quality and access in a county, we feel this combination is a functional proxy measure.
In conclusion, as local urologic cancer mortality improvements quickly plateau with increasing urologist density, a nuanced and geographically aware policy toward the size and distribution of the future urologist work force may provide the greatest population-level benefits. These findings may also have important implications for overall oncologist density and distribution.
Acknowledgment
We thank Nap Hosang, MD, MPH, MBA, and Kirk Smith, PhD, MPH of the School of Public Health, University of California, Berkeley, CA, for inspiration and discussions leading to the genesis of this investigation.
Footnotes
Supported by the National Institutes of Health and National Center for Cancer Resources Grant No. UCSF-CTSI (UL1 RR024131).
Presented in part at the Western Section American Urological Association Meeting, October 26-30, 2008, Monterey, CA; and the American Urological Association National Meeting, April 25-30, 2009, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Anobel Y. Odisho, Matthew R. Cooperberg, Peter R. Carroll
Collection and assembly of data: Anobel Y. Odisho, Ardalan E. Ahmad
Data analysis and interpretation: Anobel Y. Odisho, Matthew R. Cooperberg, Vincent Fradet, Ardalan E. Ahmad, Peter R. Carroll
Manuscript writing: Anobel Y. Odisho, Matthew R. Cooperberg
Final approval of manuscript: Anobel Y. Odisho, Matthew R. Cooperberg, Vincent Fradet, Peter R. Carroll
REFERENCES
- 1.US Census Bureau. Population Projections. http://www.census.gov/population/www/projection/index.html.
- 2.Cooper RA. The coming era of too few physicians. Bull Am Coll Surg. 2008;93:11–18. [PubMed] [Google Scholar]
- 3.Cooper RA. The future of specialty care in the US. Nat Clin Pract Rheumatol. 2008;4:333. doi: 10.1038/ncprheum0844. [DOI] [PubMed] [Google Scholar]
- 4.Macinko J, Starfield B, Shi L. Quantifying the health benefits of primary care physician supply in the United States. Int J Health Serv. 2007;37:111–126. doi: 10.2190/3431-G6T7-37M8-P224. [DOI] [PubMed] [Google Scholar]
- 5.Phillips RL, Dodoo MS, Green LA. Adding more specialists is not likely to improve population health: Is anybody listening? Health Aff (Millwood) 2005;(suppl):W5-111–W5-114. doi: 10.1377/hlthaff.w5.111. [DOI] [PubMed] [Google Scholar]
- 6.Goodman DC, Grumbach K. Does having more physicians lead to better health system performance? JAMA. 2008;299:335–337. doi: 10.1001/jama.299.3.335. [DOI] [PubMed] [Google Scholar]
- 7.Starfield B, Shi L, Grover A, et al. The effects of specialist supply on populations' health: Assessing the evidence. Health Aff (Millwood) 2005;(suppl):W5-97–W5-107. doi: 10.1377/hlthaff.w5.97. [DOI] [PubMed] [Google Scholar]
- 8.Ferrante JM, Gonzalez EC, Pal N, et al. Effects of physician supply on early detection of breast cancer. J Am Board Fam Pract. 2000;13:408–414. doi: 10.3122/15572625-13-6-408. [DOI] [PubMed] [Google Scholar]
- 9.Roetzheim RG, Pal N, Gonzalez EC, et al. The effects of physician supply on the early detection of colorectal cancer. J Fam Pract. 1999;48:850–858. [PubMed] [Google Scholar]
- 10.Ricketts TC, Holmes GM. Mortality and physician supply: Does region hold the key to the paradox? Health Serv Res. 2007;42:2233–2251. doi: 10.1111/j.1475-6773.2007.00728.x. discussion 2294-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eide MJ, Weinstock MA, Clark MA. The association of physician-specialty density and melanoma prognosis in the United States, 1988 to 1993. J Am Acad Dermatol. 2009;60:51–58. doi: 10.1016/j.jaad.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman DC, Fisher ES, Little GA, et al. The relation between the availability of neonatal intensive care and neonatal mortality. N Engl J Med. 2002;346:1538–1544. doi: 10.1056/NEJMoa011921. [DOI] [PubMed] [Google Scholar]
- 13.Onega T, Duell EJ, Shi X, et al. Geographic access to cancer care in the U.S. Cancer. 2008;112:909–918. doi: 10.1002/cncr.23229. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute, US Centers for Disease Control and Prevention. State cancer profiles. http://statecancerprofiles.cancer.gov.
- 15.US Department of Health and Human Services, Health Resources and Services Administration. Area Resource File (ARF): National county-level health resource information database. http://www.arfsys.com.
- 16.US Department of Agriculture, Economic Research Service. Rural-Urban Continuum Codes. http://www.ers.usda.gov/Data/RuralUrbanContinuumCodes/
- 17.US Census Bureau. Small-area health insurance estimates: Model-based small-area health insurance estimates for counties and states. http://www.census.gov/hhes/www/sahie.
- 18.Odisho AY, Fradet V, Cooperberg MR, et al. Geographic distribution of urologists throughout the United States using a county level approach. J Urol. 2009;181:760–765. doi: 10.1016/j.juro.2008.10.034. discussion 765-766. [DOI] [PubMed] [Google Scholar]
- 19.Allen TD, Glenn JF, Graham SD, et al. Too much of a good thing. J Urol. 1978;120:267–268. doi: 10.1016/s0022-5347(17)57136-0. [DOI] [PubMed] [Google Scholar]
- 20.Ansell JS. Trends in urological manpower in the United States in 1986. J Urol. 1987;138:473–476. doi: 10.1016/s0022-5347(17)43232-0. [DOI] [PubMed] [Google Scholar]
- 21.National Supply and Demand Forecast for Urologists 1995 to 2020. Baltimore, MD: American Urological Association; 1995. Final Report to AUA Executive Committee. [Google Scholar]
- 22.Weiner DM, McDaniel R, Lowe FC. Urologic manpower issues for the 21st century: Assessing the impact of changing population demographics. Urology. 1997;49:335–342. doi: 10.1016/S0090-4295(96)00492-X. [DOI] [PubMed] [Google Scholar]
- 23.Jemal A, Ward E, Wu X, et al. Geographic patterns of prostate cancer mortality and variations in access to medical care in the United States. Cancer Epidemiol Biomarkers Prev. 2005;14:590–595. doi: 10.1158/1055-9965.EPI-04-0522. [DOI] [PubMed] [Google Scholar]
- 24.Jemal A, Kulldorff M, Devesa SS, et al. A geographic analysis of prostate cancer mortality in the United States, 1970-89. Int J Cancer. 2002;101:168–174. doi: 10.1002/ijc.10594. [DOI] [PubMed] [Google Scholar]
- 25.Krakauer H, Jacoby I, Millman M, et al. Physician impact on hospital admission and on mortality rates in the Medicare population. Health Serv Res. 1996;31:191–211. [PMC free article] [PubMed] [Google Scholar]
- 26.Colli JL, Amling CL. Prostate cancer mortality rates compared to urologist population densities and prostate-specific antigen screening levels on a state-by-state basis in the United States of America. Prostate Cancer Prostatic Dis. 2008;11:247–251. doi: 10.1038/pcan.2008.7. [DOI] [PubMed] [Google Scholar]