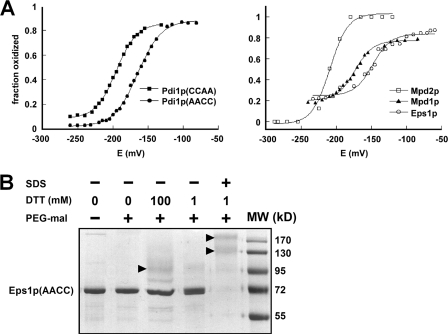
FIGURE 2.
Redox characteristics of yeast PDI family Cys-X-X-Cys motifs. A, redox potential of the samples was adjusted by varying the GSH/GSSG ratio at pH 7.0. After equilibration in redox buffer, the proteins were precipitated and reacted with mal-PEG5K, separated by SDS-PAGE, and visualized using fluorescent stain. The intensities of the bands were quantified and plotted as fraction oxidized. Data for the five Cys-X-X-Cys motifs are shown on two graphs for clarity. Each titration was fitted to the Nernst equation for a two-electron transfer to give reduction potential values of Pdi1p(CCAA): −194 mV, Pdi1p(AACC): −164 mV, Mpd1p: −174 mV, and the Eps1p amino-terminal domain: −149 mV. The Mpd2p reduction potential is estimated to be −210 mV. B, the Cys-X-X-Cys of the second Eps1p trx domain is not fully reduced by high concentrations of DTT in the folded state. Alkylation of free thiols with mal-PEG5K was used to distinguish oxidized from reduced fractions after the indicated treatments. The Eps1p(AACC) mutant was incubated with low (1 mm) or high (100 mm) DTT concentrations in the folded state or with 1 mm DTT after denaturing the protein with detergent. In the folded state, the protein was highly resistant to reduction. Only in the denatured state did substantial reduction occur, as indicated by the disappearance of the unmodified protein (labeled Eps1p(AACC) to the side of the gel) and the appearance of species of higher apparent molecular weights (indicated by arrowheads). Eps1p(AACC) contains six cysteines, so the various higher molecular weight species may have different subsets of these cysteines reduced and modified by mal-PEG5K.