FIGURE 3.
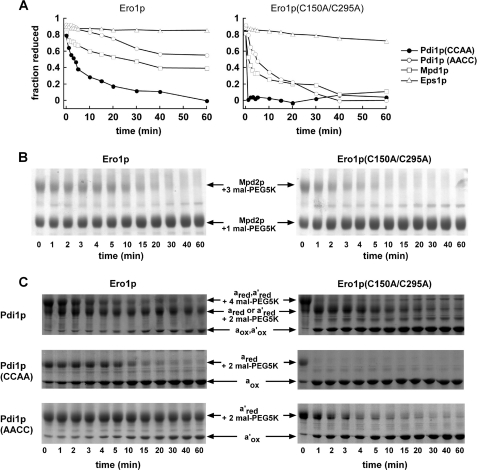
Direct oxidation rates of PDI family proteins by Ero1p monitored by gel assays. A, reduced PDI family proteins (150 μm thiols) were mixed with 2 μm Ero1p or Ero1p(C150A/C295A). Loss of reactivity with mal-PEG5K upon disulfide bond formation was monitored by quantification of the accumulating unmodified protein after separation by SDS-PAGE. Lag phases are less evident in this experiment than in Fig. 4, because of the poor time resolution of gel-based assays. Furthermore, due to the relatively low substrate/enzyme ratio in this experiment (37.5:1), a significant fraction (estimated 5–10%) of substrate is oxidized by dithiol/disulfide exchange with the Ero1p shuttle and regulatory disulfides prior to oxygen consumption and enzyme turnover. B, except for a lower Mpd2p concentration, the experiment was conducted as in A, but the gels, rather than results of quantification, are shown. Mpd2p was partially reduced by incubation with 23 mm GSH for 1 h. Following removal of GSH, Mpd2p (50 μm thiols) was mixed with 2 μm Ero1p or Ero1p(C150A/C295A). The Mpd2p protein lacking its signal sequence has seven cysteine residues. One unpaired cysteine reacts with mal-PEG5K regardless of the redox status of the Cys-X-X-Cys motif. C, the two Pdi1p active sites appear to function independently during oxidation by Ero1p, because the two oxidation events seen for wild-type Pdi1p (top panel) occur at similar rates as the oxidation of the Pdi1p(CCAA) (middle panel) and Pdi1p(AACC) (bottom panel) mutants. This observation holds for both wild-type Ero1p and the C150A/C295A mutant. Bands are labeled according to the state of each Cys-X-X-Cys motif present (subscript “red” indicates reduced and “ox” oxidized) and the number of mal-PEG5K additions present. The gels shown here, which are similar to those used to obtain the data in A, further demonstrate that only a single disulfide was reduced initially in Pdi1p(AACC) and Pdi1p(CCAA), and two disulfides in wild-type Pdi1p, indicating that the Cys-X6-Cys motif remained oxidized as intended.