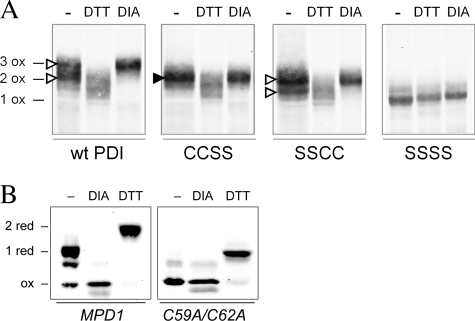
FIGURE 5.
Redox states of Pdi1p and Mpd1p in vivo. A, wild-type Pdi1p and mutants Pdi1p(CCSS), Pdi1p(SSCC), and Pdi1p(SSSS) were purified from yeast cell lysates that had been reacted with NEM to block free thiols. Purified proteins were subsequently reduced with DTT and modified by mal-PEG2K. Pdi1p was visualized by Western blotting with anti-Pdi1p serum. With this protocol, the presence of a disulfide bond in the original cell lysate is reflected by mal-PEG2K modification and a corresponding decreased migration during SDS-PAGE. Fully oxidized Pdi1p controls (e.g. three disulfide bonds for wild-type Pdi1p (3 ox)) were prepared by lysis of cells in the presence of 10 mm diamide (DIA). Reduced migration controls were prepared by cell lysis in the presence of DTT; note that these lysis conditions did not result in reduction of the structural disulfide in Pdi1p as evident by the co-migration of the DTT and diamide-treated samples for Pdi1p(SSSS). The filled arrowhead indicates the single band representing a species with an oxidized a domain in the mutant lacking the active-site cysteines of the a′ domain. The open arrowheads indicate the two bands corresponding to the oxidized and reduced forms of the a′ domain in the mutant lacking the active-site cysteines of the a domain. B, yeast mpd1Δ cells containing plasmids encoding Myc-tagged Mpd1p or an Mpd1p active-site mutant, Mpd1p(C59A/C62A), were grown in SMM at 30 °C. The oxidation state of Mpd1p was assessed after resolving mal-PEG2K-modified cellular proteins by reducing SDS-PAGE and immunoblotting with anti-Myc. Using this protocol, free cysteine thiols within Mpd1p in the original cell lysate result in mal-PEG2K modification and a corresponding decrease in migration. Oxidized and reduced controls were prepared by treatment of cell lysates with diamide or DTT prior to mal-PEG2K.