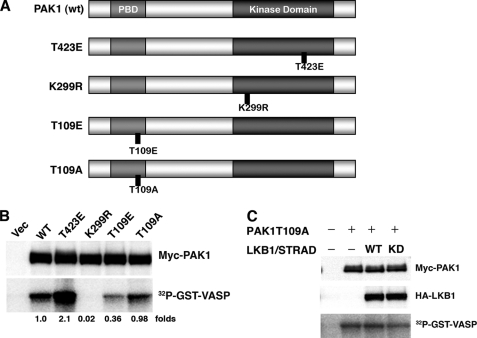
FIGURE 5.
Phosphorylation of PAK1 at Thr109 reduces its activity. A, structures of PAK1 constructs. wt PAK1 contains the p21-binding domain (light gray) and the kinase domain (dark gray). The noncatalytic domains are in white. Also displayed are the constitutively active mutant (T423E), dominant-negative mutant (K299R), phosphorylation-mimicked mutant for Thr109 (T109E), and the nonphosphorylatable mutant for Thr109 (T109A). B, the kinase activities of PAK1 mutants. The HEK293T cells were co-transfected with the indicated constructs. At 24 h post-transfection, the immunoprecipitates were prepared with an anti-Myc antibody. The precipitates were used for in vitro kinase assays using GST-VASP-(158–277) as a substrate. Cellular levels of Myc-PAK1 were determined by Western blotting with anti-Myc antibody. C, LKB1 (wt or kinase-dead (KD)) failed to inhibit the activity of the PAK1-T109A mutant. Cultured HEK293T cells were co-transfected with the indicated constructs. The kinase activities were determined as described under “Experimental Procedures.” Cellular levels of Myc-PAK1 and HA-LKB1 were determined by Western blotting with anti-Myc or anti-HA antibody. Similar results were obtained in three independent experiments. Vec, vector.