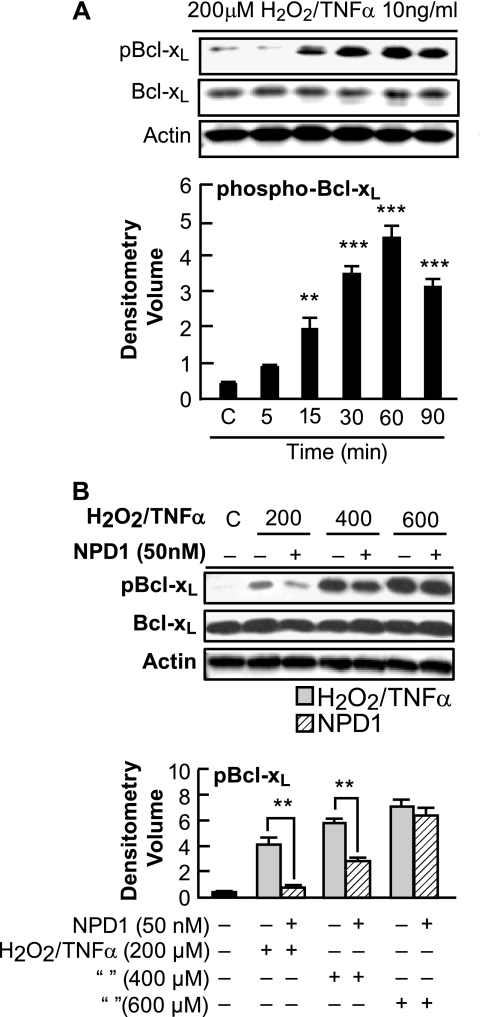
FIGURE 1.
Analysis of Bcl-xL protein phosphorylation. A, phosphorylation of Bcl-xL protein at residue Ser-62 in ARPE-19 cells in response to oxidative stress. ARPE-19 cells were serum-starved for 18 h and treated with 200 μm H2O2/TNFα for 5, 15, 30, 60, and 90 min. Immunoblot represents S62Bcl-xL, total Bcl-xL, and actin. Phospho-Bcl-xL was significantly up-regulated at 30 min. Error bars indicate ± S.E. of three sets of different experiments. ***, p < 0.001, **, p < 0.01, when compared with stress-treated cells. C indicates control. B, NPD1 down-regulated the oxidative stress-induced phosphorylation of Bcl-xL at residue Ser-62. ARPE-19 cells were serum-starved for 18 h and then induced with 200, 400, and 600 μm H2O2 and 10 ng/ml TNFα in the presence and absence of NPD1 (50 nm) for 30 min. Whole cell extracts were prepared and tested by Western blot for the presence of S62Bcl-xL and total Bcl-xL. Also, actin was used as a loading control. Phosphorylation of Bcl-xL was significantly down-regulated in the presence of 200 μm H2O2/TNFα. No statistical difference was observed in cells treated with 600 and 800 (data not shown) μm H2O2/TNFα. Error bars indicate ± S.E. of three independent experiments. **, p < 0.01, when compared with stress-treated cells.