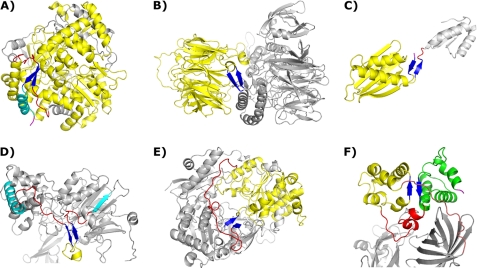
FIGURE 1.
Representative two-stranded β-sheets in the dataset. Common coloration: two-stranded β-sheets are shown in blue, intervening residues are shown in yellow, relevant stretches of coil are shown in red, and relevant chain termini are shown in magenta. A, shown is a two-stranded β-sheet encompassing multiple modules. This sheet has the largest strand separation (499 residues), and the intervening residues compose the majority of the folded protein. One strand of the sheet is in the C-terminal region followed by a final helix (cyan); the other occurs in the midst of a long coil region (PDB code 1HSS). B, module capping, with intervening residues forming a large β-propeller, is shown (PDB code 1K32). C, module capping involving chain termini is shown (PDB code 2CVE). D, shown is a two-stranded β-sheet in a stretch of extended coil transiting between two distinct modules. The final helix of one module and the initial strand of the next are shown in cyan (PDB code 1PNK). E, shown is a two-stranded β-sheet with an intervening structural module that has different entry/exit points. In this case a long extended coil region connects the entry and exit points together, allowing for sheet formation (PDB code 1DMR). F, shown is the only case of a two-stranded β-sheet composed of strands in different structural modules, not involving a chain terminus. The second intervening module is shown in green. The coil region joining the two intervening modules is shown in red, and the final exiting coil stretch joining these modules to the rest of the protein is shown in salmon (PDB code 1PBY).