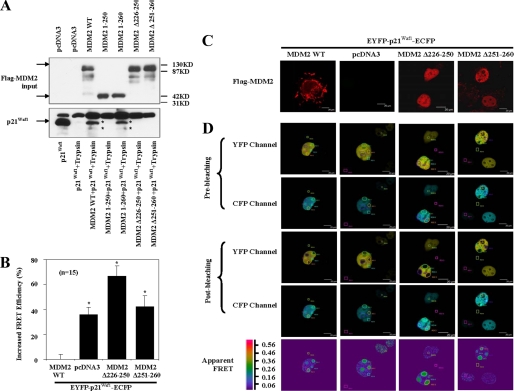
FIGURE 5.
MDM2 (aa 251–260) induces changes in p21Waf1 conformation. A, p21Waf1 and MDM2 or MDM2 deletions were incubated with trypsin in vitro for 35 min at 20 °C. The proteolytic profiles of p21Waf1 were determined by immunoblotting with p21Waf1 antibodies (Ab-1, Calbiochem; H-164, 187, and F-5, Santa Cruz Biotechnology). B, FRET efficiencies from COS7 cells transfected with EYFP-p21Waf1-ECFP or from cells transfected with combinations of MDM2, MDM2Δ226–250, or MDM2Δ251–260 with EYFP-p21Waf1-ECFP were measured in 15 cells from each group and are representative of five distinct experiments. The increases in relative FRET efficiency are shown in the figure (*, p < 0.05). Error bars represent S.D. C and D, COS7 cells were transfected to overexpress proteins as in the above panels. C, the subcellular localization of the transfected wild-type and mutant MDM2 proteins was determined by immunofluorescence. D, FRET was measured as the ratiometric increase in ECFP fluorescence upon photobleaching of EYFP. ECFP and EYFP images were taken both before and after acceptor photobleaching. The areas of photobleaching are labeled as regions of interest (ROI) 1, 2, and 3. The areas of the background are labeled as regions of interest 4, 5, and 6. FRET efficiency was displayed as a pseudocolor representation. Scale bar, 20 μm. WT, wild-type.