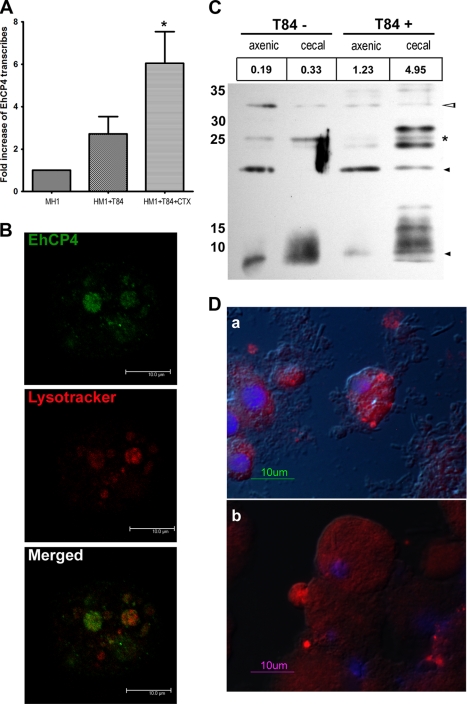
FIGURE 5.
Endogenous EhCP4 expression and release. A, up-regulation of ehcp4 mRNA following co-culture with T84 cells. Quantitative reverse transcription-PCR was performed on E. histolytica in culture medium alone, co-cultured with T-84 cells alone, and co-cultured with T-84 cells pretreated by cholera toxin to stimulate mucin secretion (*, p = 0.03, Student's t test). B, EhCP4 localizes to acidic vesicles. An optical section of an E. histolytica trophozoite stained with anti-EhCP4 antibody (green) and LysoTrackerTM Red DND-99 (red) showed EhCP4 localized to acidic vesicles, following co-culture with T84 monolayers for 2 h. Z-Stack thickness was 0.5 μm, scan depth was 10.1 μm, and scale bar is 10 μm. C, Western blot of EhCP4 released. CM proteins from 8.8 × 105 trophozoites were trichloroacetic acid-precipitated and resolved with a 15% SDS-polyacrylamide gel. EhCP4 protein release was detected in CM from E. histolytica trophozoites in medium alone (T84−) and from trophozoites following an overnight co-culture with a T84 monolayer (T84+). CM from axenically cultured E. histolytica trophozoites and the CM from trophozoites that infected mouse ceca are indicated as axenic and cecal, respectively. The numbers (RFU/min) above the lanes indicate the proteinase activity represented by Z-VVR-AMC. Hollow arrowhead, pro-EhCP4 (∼32 kDa); star, mature EhCP4 (∼26 kDa); black arrowhead, degraded EhCP4. D, EhCP4 release in vivo. EhCP4 (red) is shown in vesicles on the trophozoite surface by immunofluorescence staining with anti-EhCP4 antibody. Sections (8 μm) are from mouse ceca 1 week postinoculation (a) and 2 weeks postinoculation (b), respectively. Images were obtained by epifluorescence microscopy overlaid with differential interference contrast image.