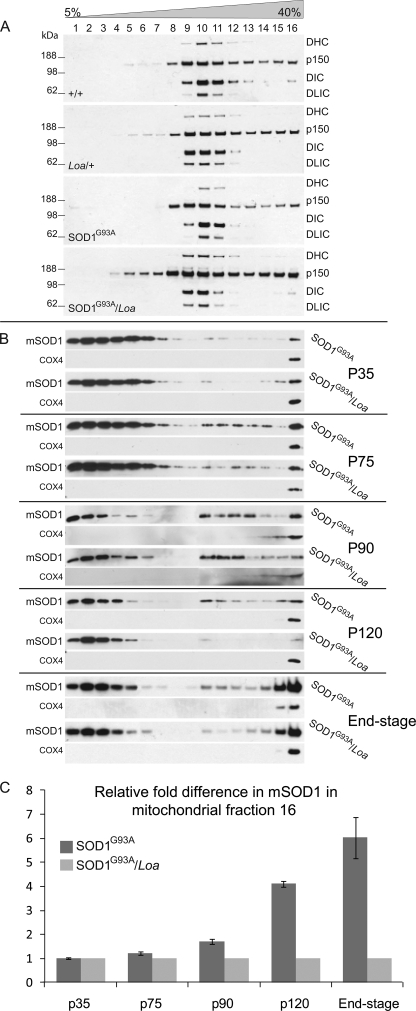
FIGURE 1.
The integrity and sucrose sedimentation pattern of the dynein complex is not compromised in SOD1G93A and SOD1G93A/Loa, but the Loa mutation in dynein alters the sedimentation of mutant SOD1 in SOD1G93A/Loa mice. A, brain homogenates of equal protein contents prepared from +/+, Loa/+; SOD1G93A, and SOD1G93A/Loa mice (at end stage of disease in the SOD1G93A and SOD1G93A/Loa mice) were sedimented in 5–40% continuous sucrose gradient by centrifugation at 237,000 × g for 4 h at 4 °C as described under “Experimental Procedures.” Then 16 equal-volume fractions. were collected from top to bottom and analyzed by SDS-PAGE and Western blotting detection for DHC, p150, DIC, and dynein light intermediate chain (DLIC) as representatives of the dynein-dynactin complex. B, shown are brain homogenates of equal protein contents prepared from SOD1G93A and SOD1G93A/Loa littermates at the postnatal stages; P35 (before disease onset), P75 (onset of disease), P90 (early stage of disease), P120 (late stage of disease), and end-stage, were sedimented in 5–40% continuous sucrose gradient by centrifugation at 237,000 × g for 4 h at 4 °C as described under “Experimental Procedures.” Then 16 equal-volume fractions were collected from top to bottom and analyzed by SDS-PAGE and Western blotting detection for SOD1 (to avoid overexposure caused by much higher levels of mutant SOD1 (mSOD1) protein in the lighter fractions of the gradient, we loaded 3 μl of fractions 1–3, 8 μl of fractions 5–7, 10 μl of fractions 8–9, and 20 μl of fractions 11–16; supplemental Fig. 3 shows the true representations of equal loading of the fractions on SDS-PAGE immunoblots). C, -fold difference (n = 3–6) in mutant SOD1 protein in fraction 16 of the SOD1G93A mice was quantified relative to that in SOD1G93A/Loa mice using COX4 as the internal control.