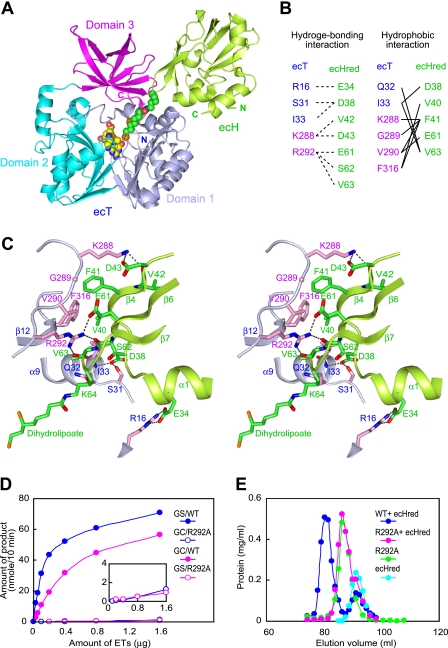
FIGURE 2.
Interface structure of the heterodimer of the ecT·ecHred complex. A, schematic representation of the ecT·ecHred heterodimer (MolAE). Domains 1, 2, and 3 of ecT are colored in light blue, cyan, and magenta, respectively, and ecHred is shown in green with the dihydrolipoyllysine arm shown in CPK model colored in green (carbon), blue (nitrogen), red (oxygen), and orange (sulfur). The bound 5-CH3-THF is also shown in a CPK model colored in yellow (carbon) and the same colors for other atoms as for dihydrolipoyllysine. B, residues contributing to the ecT and ecHred interface. Hydrogen bonding and hydrophobic interactions are summarized. C, close-up view of the interface of molAE in stereo. Key residues from ecT (molA) and ecHred (molE) are shown as sticks with carbon atoms colored in light blue and pink for ecT and green for ecHred. The dihydrolipoyllysine arm is also in stick colored as in A. Hydrogen bonds are depicted as broken lines. D, glycine cleavage (GC) and synthesis (GS) activities of wild-type (WT) and R292A mutant ecT in the overall reaction assays. The inset depicts the trace activities by a large amount of the mutant. E, evaluation of the heterodimer-forming ability of ecT mutant. ecT or ecTR292A was mixed with 1.5–2-fold molar excess of ecHred and subjected to gel filtration. ecT was eluted at the same position of ecTR292A.