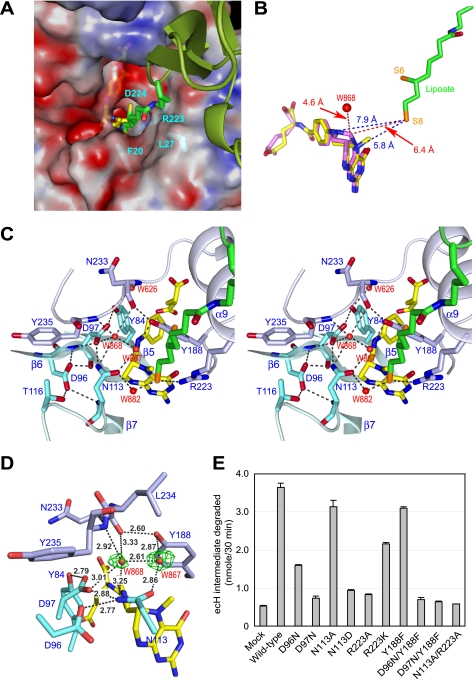
FIGURE 3.
Active site of ecT in complex with ecHred and 5-CH3-THF. A, molecular surface of the active site pocket of ecT with the dihydrolipoyllysine arm inserted. Red and blue surfaces indicate negatively and positively charged areas, respectively. ecH is shown schematically, colored in green, and the dihydrolipoyllysine and 5-CH3-THF are in stick colored as in Fig. 1A. The residues of ecT interacting with the dihydrolipoyllysine arm were shown in stick with labels. B, distances between the tip of dihydrolipoate and the atoms of folate cofactors. 5,10-CH2-THF (magenta) was modeled based on the structure of 5-CH3-THF (yellow). Distances (Å) are shown with broken lines. C, close-up view of the active site in stereo. Key residues and four water molecules are depicted in stick (cyan and light blue) and sphere (red), respectively, with labels. The dihydrolipoyllysine and 5-CH3-THF are as in A, and hydrogen bonds are drawn as broken lines. D, putative ammonium-binding site in the reverse reaction. Omit Fo − Fc electron density for the water molecules at the active site of molAE is shown in green, contoured at 2.0σ. Well ordered W868 may occupy the ammonium-binding site. E, ecHint cleavage activity of wild-type and mutant ecTs in the absence of THF. The reaction was carried out as described under “Experimental Procedures.” Mock means nonenzymatical degradation of ecHint. Error bars show variability among three independent measurements.