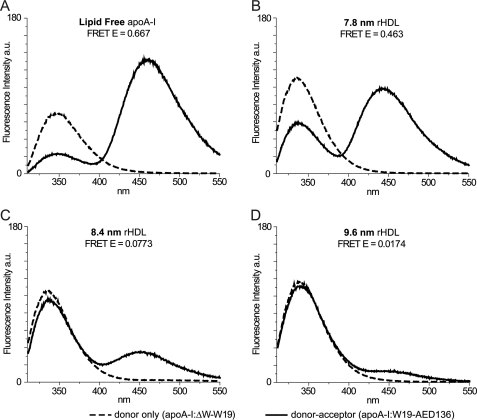
FIGURE 4.
Fluorescence spectra of protein variants. Sample concentrations were 0.25 mg/ml; excitation wavelength was 280 nm; and emission was from 310 to 550 nm. The bandwidth of excitation and emission monochromators was 1.5 nm. Emission of the single Trp at position 19 of apoA-I:ΔW-W19 (donor-only) is reported as a dashed line for lipid-free (A) and each lipidation state (7.8-nm(B), 8.4-nm (C), and 9.6-nm (D) rHDL). Background fluorescence from Tyr was eliminated by subtracting the emission spectrum of apoA-I:ΔW (Trp-null background) from the emission spectrum of apoA-I:ΔW-W19 (donor-only) in the same lipidation state. The solid lines are emission spectra of Trp and AEDANS in apoA-I:W19-AED136 (donor-acceptor). Acceptor fluorescence is due to energy transfer alone; Tyr background and direct excitation of AEDANS were eliminated by subtracting the emission spectra of apoA-I:ΔW-AED136 (acceptor-only in Trp-null background) from the emission spectra of apoA-I:W19-AED136 in the same lipidation state. Energy transfer efficiency was calculated from background-corrected spectra by comparing Trp fluorescence intensities (integration over 310–395 nm) of apoA-I:ΔW-W19 (donor-only) and apoA-I:W19-AED136 (donor-acceptor).