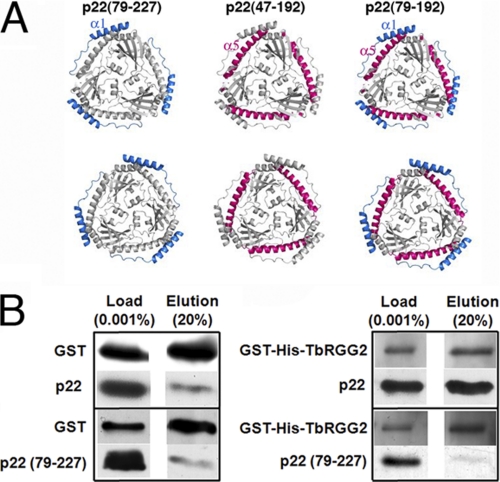
FIGURE 8.
Recombinant p22 and the accessory factor TbRGG2 directly interact in vitro and p22 residues 47–78 are critical for this interaction. A, p22 structure showing the regions that were removed to examine their roles in TbRGG2 binding by GST-pulldown studies. The leftmost p22 shows the location of residues 47–78 (colored blue), the middle shows the location of the C-terminal residues, 193–227, that were removed (colored red), and the final figure (rightmost) shows the relative location of both truncated regions. Only p22(79–227) was successfully expressed and purified and therefore tested. B, purified GST tag alone (3 μm) or GST-His-TbRGG2 (300 nm) were incubated with 300 nm full-length (p22) or truncated p22 (p22(79–227)) in the presence of glutathione-agarose beads. Left: results using the GST protein alone. The input proteins are shown in the Load lane. Following binding and subsequent washes, bound proteins were eluted from the beads with excess free glutathione. Twenty percent of the total elution volume was analyzed by Western blotting for GST and the p22 proteins. Right: results as in A, except using recombinant GST-His-TbRGG2.