Abstract
Granule-associated perforin and granzymes (gzms) are key effector molecules of cytotoxic T lymphocytes (Tc cells) and natural killer cells and play a critical role in the control of intracellular pathogens and cancer. Based on the notion that many gzms, including A, B, C, K, H, and M exhibit cytotoxic activity in vitro, all gzms are believed to serve a similar function in vivo. However, more recent evidence supports the concept that gzms are not unidimensional but, rather, possess non-cytotoxic potential, including stimulation of pro-inflammatory cytokines and anti-viral activities. The present study shows that isolated mouse gzmB cleaves the actin-severing mouse protein, cytoplasmic gelsolin (c-gelsolin) in vitro. However, when delivered to intact target cells by ex vivo immune Tc cells, gzmB mediates c-gelsolin proteolysis via activation of caspases 3/7. The NH2-terminal c-gelsolin fragment generated by either gzmB or caspase 3 in vitro constitutively severs actin filaments without destroying the target cells. The observation that gzmB secreted by Tc cells initiates a caspase cascade that disintegrates the actin cytoskeleton in target cells suggests that this intracellular process may contribute to anti-viral host defense.
Keywords: Apoptosis, Calcium/Binding Protein, Cell/Apoptosis, Cytoskeleton/Actin, Immunology, Protease/Caspase, Protease/Serine Protease
Introduction
Cytotoxic T lymphocytes (Tc cells)6 and natural killer (NK) cells are indispensable for effective control of intracellular pathogens, primarily viruses, and neoplasms (1). After engaging a target cell, both cytotoxic effector cells act through either the death receptor pathway (i.e. Fas/FasL) and/or the granule secretory pathway. In the latter instance, perforin (perf) and a family of serine proteases, granzymes (gzms), are secreted by Tc/NK cells to kill target cells (2). Isolated gzmA and gzmB as well as the less-expressed gzmC, gzmH, gzmK, and gzmM, all induce apoptosis in the presence of perf (3). However, the finding that the concerted action of perf with gzmA and gzmB is essential for the control of many viruses and tumors leads to the prediction that Tc/NK cells are primarily cytotoxic executioners in vivo (4). Although this notion appears valid for gzmB, the data for gzmA and gzmA+ Tc cells are conflicting (3, 5). Recent studies indicate that isolated human and mouse gzmA are not cytotoxic at nanomolar concentrations, which are sufficient for gzmB-induced apoptosis (6). Thus, isolated human gzmA as well as human NK cells selectively delivering gzmA were shown to induce human monocytes to secrete interleukin-1β and tumor necrosis factor α in a caspase 1-dependent manner. Similarly, isolated mouse gzmA and ex vivo virus-immune mouse gzmA+ Tc cells were found to induce interleukin-1β release from primary mouse macrophages, and gzmA KO mice were shown to resist lipopolysaccharide-induced toxicity (6).
Our recent finding that ex vivo derived, virus-immune gzmB+ Tc (gzmA−/− Tc) cells induce cell death, even after blockade of caspase activation and normalization of mitochondrial events (mitochondrial depolarization, reactive oxygen species production, and cytochrome c release) suggests the existence of an additional gzmB-dependent death pathway (7). Although numerous cytoplasmic and nuclear substrates have been described for gzmB (3), how the cleavage of these proteins contribute to Tc cell cytotoxicity is poorly understood. Nevertheless, the putative involvement of the actin cytoskeleton in gzmB-mediated apoptosis has been implicated, although not formally proven, by two recent observations. First, gzmB was reported to directly cleave α-tubulin, a major component of the cell cytoskeleton, leading to perturbation of microtubule networks during Tc cell-mediated cell death (8–10). Second, FasL/Fas-mediated apoptosis is associated with the cleavage of the cytoskeletal protein, gelsolin (c-gelsolin), by caspase 3, producing morphological alterations in the target cell (11). Together with the concept that gzmB expresses an aspartase activity similar but not identical to caspase 3 (12), these results led us to speculate that c-gelsolin might also be a physiological substrate of gzmB in Tc cell-mediated processes.
C-gelsolin is a highly conserved, calcium (Ca2+)-regulated member of a family of actin-binding proteins (13). Initially identified in the cytoplasm of many vertebrate cells, the protein consists of six homologous domains (S1-S6) combined into an NH2- (S1-S3) and a COOH-terminal (S4-S6) half. In its inactive, Ca2+-free state, c-gelsolin exists in a closed and compact conformation tied together by a COOH-terminal helix, which is non-covalently bound to the S2 domain. C-gelsolin has been implicated in a number of intracellular processes, including regulation of cell morphology, motility, signaling, and apoptosis (13, 14). The most extensively studied function of the c-gelsolin isoform is its actin filament modifying activity (13, 15). Ca2+ binding leads to a structural rearrangement in the NH2- and COOH-terminal halves of the molecule that allows severing and capping of actin (15). During Fas-mediated apoptosis, c-gelsolin is cleaved by caspase 3 to generate an NH2-terminal fragment that functions independently of Ca2+ to disrupt cell architecture (11). Besides modulating cytoskeletal dynamics, c-gelsolin also regulates signal transduction through the adhesion molecule E-cadherin and the Ras/Rac pathway (16) and was reported to activate DNase I, a key enzyme for DNA fragmentation in apoptosis (17). However, the impact of the differential biological activities of native and fragmented c-gelsolin observed on gzmB-mediated apoptosis remains controversial (11, 18, 19).
To further understand the biological role of gzmB in Tc cell-mediated processes, we have analyzed whether c-gelsolin is a physiological substrate for gzmB. We show here that although isolated gzmB cleaves c-gelsolin in vitro, processing induced by Tc cells is mediated instead by caspases 3/7, activated via gzmB. However, in both cases disruption of actin filaments is observed. Thus, gzmB+ Tc cell-induced disintegration of the actin cytoskeleton may represent an important host defense mechanism against intracellular pathogens.
EXPERIMENTAL PROCEDURES
Mouse Strains
The following strains were maintained at the Max-Planck-Institute of Immunobiology, Freiburg. C57BL/6 (B6) and strains deficient for gzmA (gzmA−/−), gzmB (gzmB−/−), gzmA and gzmB (gzmA×B−/−), perf, gzmA and gzmB (perfxgzmAxB−/−), and mutated FasL gene (gld/gld) were bred on the B6 background (20). A mouse strain deficient for gelsolin (gelsolin−/−) was bred on a mixed 129/Sv x Balb/c background (21). For the detection of the gelsolin mutation, DNA was analyzed by PCR using the following primers: Gelsolin +1, 5′-GTG GAC CAC CCC GAA TT-3′; Gelsolin +267, 5′-CTC AGT TCA GGT ATA TCC ATA CAG TT-3′; Neo-3, 5′-ATT GAA CAA GAT GGA TTG CAC-3′; Neo-4, 5′-CGT CCA GAT CAT CCT GAT C-3′. Mice of the same sex at 8 to 12 weeks of age were used in all experiments and were conducted in accordance with the ethical guidelines of the Federation of European Laboratory Animal Science Association.
Cell Lines, Cell Culture, and Reagents
SV40-transformed mouse embryonic fibroblasts (MEF) wt cells were provided by Christoph Borner. Casp 3−/−, casp 7−/−, and casp 3 × 7−/− MEFs were obtained from R. Flavell (22) and compared with a MEF wt cell line generated by the same group. MEFs, EL4.F15, MC.Fas−/− (23), and RMA (24) cells were cultured in minimal essential medium (PAA Laboratories) supplemented with 10% heat-inactivated fetal calf serum (Biochrom AG), 2-mercaptoethanol (10−5 m, Sigma), 10 μg/ml tylosin (Sigma) and 10 μg/ml kanamycin (Invitrogen) at 37 °C, 7% CO2. For culture of 1.3E6SN cells, medium was supplemented with 10% concanavalin A supernatant + 20 mg/ml α-methyl-d-mannopyranoside (23). For MBL-2.Fas cells (25), medium was supplemented with 1 mg/ml G418 (Calbiochem). The MC.Gelsolin−/− cell line was generated as described for MC.Fas−/− (23) and cultured as described above.
Proteins and Plasmids
Mouse c-gelsolin cDNA was amplified by PCR with Vent polymerase (New England Biolabs) from reverse-transcribed mRNA isolated from EL4.F15 cells. Primers were constructed from the c-gelsolin sequence to contain BamHI restriction sites, and the PCR product was ligated in-frame into pGEX-2T (Amersham Biosciences), which includes the glutathione S-transferase gene at the NH2 terminus of the expressed recombinant (rec.) fusion protein. Plasmid expression of transfected colonies of JM109 (Promega), affinity purification on glutathione-Sepharose column, and endoproteinase thrombin cleavage of the glutathione S-transferase fusion protein was performed as recommended by the manufacturer (Amersham Biosciences). Protein concentration of c-gelsolin was determined by BCA assay (Pierce). Rec.gzmB and pro-gzmB were generated as described before (23). Rec.caspase 3 was purchased from Axxora GmbH. The full-length cDNA fragment of c-gelsolin was cloned by PCR, ligated into expression vector pcDNA 3.1/V5-His (Invitrogen), and used for transfection of MC.Gelsolin−/− cells.
Cleavage of Purified Gelsolin or Gelsolin in Cell Lysates
300 nm purified rec.c-gelsolin or cell lysates without protease inhibitors were incubated with the indicated concentrations of proteases for the indicated time points in 20 μl of gelsolin reaction buffer (for gzmB: 6 mm Tris/HCl, 1.2 mm CaCl2, pH 7.5; for caspase 3: 6 mm Tris/HCl, 1.2 mm CaCl2, 1.5 mm MgCl2, 1 mm KCl, pH 7.5). For specific inhibition of proteases, they were preincubated with 100 μm appropriate inhibitor at 37 °C for 30 min and added to purified gelsolin or cell lysates. Reactions were terminated by adding 10 μl of 3× reducing SDS-sample buffer and boiling at 95 °C for 5 min. The reaction products were subjected to SDS-PAGE.
Determination of Cleavage Sites
To determine the cleavage sites in rec.c-gelsolin generated by rec.gzmB, 100 pmol of gelsolin were incubated with 100 pmol of active protease or with 100 pmol of protease, inactivated at 37 °C for 30 min by 100 μm dichloroisocoumarin (DCI) (gzmB) before using. The cleavage took place in gelsolin reaction buffer for gzmB in a total volume of 100 μl at 37 °C for 3 h. 3 μl of each sample were used for Western blot to ascertain that the cleavage worked. Liquid chromatography/electrospray-mass spectrometry (LC/ES-MS) analysis was performed by using the high performance liquid chromatography ETTAN-LC (GE Healthcare) coupled to a quadrupole time-of-flight spectrometer (Waters). 40 pmol of the different experimental set-ups were injected on a PLRP-S 300 Å 3-μm 150 × 2.1-mm column (Polymer Laboratories). A linear gradient from 0% to 100% in 50 min with a flow rate of 200 μl/min was performed (buffer A: 0.05% formic acid in water; buffer B: 0.05% formic acid in 90% acetonitrile). The Q-ToF was scanned from 400 to 3000 m/z (mass-to-charge ratio). Manual transformation and the MaxEnt1 algorithm (Waters) was used to deconvolute the multiple charged signals in the averaged spectra.
Actin Depolymerization Assay
Pyrene muscle actin (Actin Polymerization Biochem kit; Cytoskeleton) was dissolved according to the manufacturer's instructions to a concentration of 0.1 mg/ml actin, and the mix was left on ice for 1 h to depolymerize actin oligomers. The solution was centrifuged, and 200 μl (20 μg of actin) were polymerized according to manufacturer's instructions. 8 μg of intact rec.c-gelsolin, cleaved c-gelsolin, protease alone, or buffer (for gzmB: 6 mm Tris/HCl, 1.2 mm CaCl2, pH 7.5; for caspase 3: 6 mm Tris/HCl, 1.2 mm CaCl2, 1.5 mm MgCl2, 1 mm KCl, pH 7.5) were added in a volume of 20 μl to the polymerized actin. The polymerization and depolymerization were monitored by measuring the fluorescence (excitation, 350 nm; emission, 407 nm) every 60 s.
Generation of ex Vivo Lymphocytic Choriomeningitis Virus (LCMV)-immune CD8+ Cells
Mice were infected with 1 × 105 plaque-forming units of LCMV-WE intraperitoneally according to established protocols (7). At day 8 post-infection, spleens were removed, and CD8+ cells were positively selected using anti-CD8-MicroBeads (Miltenyi Biotec) with an autoMACS (Miltenyi Biotec) according to the manufacturer's instructions and stored at 4 °C before using in cytotoxic assays or for phenotypical analysis.
Cytotoxicity Assays
All cytotoxicity assays were performed in cell culture medium in which fetal calf serum was replaced by bovine serum albumin (Sigma) employing day 8 post-infection LCMV-immune CD8+ cells as effectors and target cells previously incubated (37 °C for 1 h) with 1 μm synthetic peptide KAVYNTATC (Neosystem) derived from the glycoprotein of LCMV (gp33) as described before (23). For the inhibition of caspase activity, target cells were preincubated for 1 h with the following peptide inhibitors diluted in DMSO: zDEVD-cmk (100 μm) or zVAD-fmk (100 μm). In other experiments, 5 mg/ml anti-FasL monoclonal antibody (mAb) (clone Jo2; BD Pharmingen) or the appropriate control antibody (hamster IgG; BD Pharmingen) or sublytic concentrations of streptolysin (SLO) (26) together with rec.gzmB were added to cell cultures and incubated at 37 °C and 7% CO2 for the indicated time points.
Analysis of Pro-apoptotic Processes
After treatment of target cell lines, apoptotic parameters for cell membrane pertubation were tested in the target population (CD8− in the case of incubation with Tc cells) by fluorescence-activated cell sorting (FACS) with FACSCalibur and analyzed with CellQuest. Phosphatidylserine (PS) exposure and propidium iodide (PI) uptake were analyzed by FACS using the annexin V-fluorescein isothiocyanate/allophycocyanin apoptosis detection kit from BD Pharmingen.
Detection of Gelsolin or Caspase 3 Cleavage Pattern by Western Blot Analysis or Silver Staining
Samples of in vitro cleavage assays or cell lysates, generated after cytotoxicity assays, were subjected to Western blot analysis (12.5% SDS-PAGE) under reducing conditions. The anti-native (nat.) bovine plasma gelsolin IgG, used for detection of purified gelsolin, was generated by immunizing a rabbit with nat. bovine plasma gelsolin (Sigma). The anti-rec.c-gelsolin immune serum (IS) that detects gelsolin in lysates was generated by immunizing a rabbit with rec.c-gelsolin. Anti-human caspase 3 IgG (active and inactive) was from Cell Signaling Technology. Blots were then stained with horseradish peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc.) followed by enhanced chemiluminescence (ECL) (ECL Western blotting Analysis System, GE Healthcare). Analysis of cleaved rec.c-gelsolin was performed with Silver Stain (Bio-Rad).
Transfection of Cells
MC.Gelsolin−/− cells were transfected with different plasmids using Lipofectamine 2000 (Invitrogen) according to manufacturer's instructions. The cells were incubated at 37 °C and 7% CO2 for 18 h before testing the gene expression by either intracellular FACS staining or immunofluorescence staining. For staining the cells with the in situ cell death detection kit TMR red (Roche Applied Science), cells transfected with the vector for 18 h were treated with 5 μm staurosporine (Sigma) at 37 °C and 7% CO2 for 4 h before immunofluorescence staining.
Staining for Fluorescence Microscopy
MC.Gelsolin−/− or MEF wt cells were seeded onto 12 mm poly-l-lysine-coated coverslips (BD BioCoat) in 24-well plates. After transfection or a cytotoxicity assay, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% saponin. The cells were stained with anti-rec.c-gelsolin IS diluted in saponin buffer/1× Roti-Block (Roth) at room temperature for 45 min followed by incubation with an anti-rabbit IgG Alexa 488 (Invitrogen) or phalloidin Alexa Fluor 546 (Invitrogen) diluted in saponin buffer/1× Roti-Block at room temperature for 45 min. After washing, coverslips were embedded with Fluoromount-G containing Hoechst 33342 (10 μg/ml; Invitrogen) and analyzed by fluorescence microscopy using a Zeiss microscope (Imager.Z1 Axio with Axiocam; Zeiss) and Zeiss AxioVision Rel 4.7.2 as software. For staining with the in situ cell death detection kit TMR red, MC.Gelsolin−/− cells were treated and stained with anti-rec.c-gelsolin IS and anti-rabbit IgG Alexa488 as described above followed by staining with the in situ cell death detection kit TMR red according to manufacturer's instructions. For the negative or positive control, the fixed and permeabilized cells were incubated without the terminal deoxynucleotidyltransferase instead of the TUNEL (terminal deoxyribonucleotidyltransferase-mediated dUTP nick end labeling) reaction mixture or with 1 unit of rec.DNase I (Invitrogen) at room temperature for 10 min before to labeling procedures, respectively. Coverslips were embedded and analyzed as described above.
Flow Cytometry
Analyses using FACS were done as previously described (7, 27) using fluorescein isothiocyanate- and allophycocyanin-labeled anti-CD8a mAb (clone 53-6.7; BD Pharmingen), fluorescein isothiocyanate-labeled anti-Fas mAb (clone Jo2; BD Pharmingen) for surface staining diluted in an anti-Fc receptor mAb (clone 2.4G2) and anti-nat.gzmA IgG (27), anti-rec.gzmB IgG (23), anti-human caspase 3 mAb fluorescein isothiocyanate (clone C92–605; BD Pharmingen), and anti-rec.c-gelsolin IS for intracellular staining diluted in permeabilizing buffer (phosphate-buffered saline, 5% fetal calf serum, 0.1% NaN3, 0.1% saponin (Roth)). As detection antibodies, Alexa 647-labeled goat anti-rabbit IgG was used, and as isotype controls, rabbit IgG (Jackson ImmunoResearch Laboratories Inc.) was used.
Probing for mRNA Transcription
Total RNA was extracted from up to 5 × 106 cells using the QIAshredder spin columns, the RNeasy Mini kit, and the RNase-free DNase kit (all from Qiagen) according to manufacturer's instructions, and specific transcripts were amplified. The sense/antisense primers for Gzma, Gzmb, Gzmc-Gzmg, Gzmk, Prf1, and Hprt1 (MWG) were described before (7, 27, 28). The PCR products were analyzed by gel electrophoresis (2% agarose), stained with ethidium bromide, and analyzed using an UV-transluminator (Vilber Lourmat). A 100-bp DNA size marker was used (Invitrogen).
RESULTS
Cleavage of Rec. and Nat. c-gelsolin by rec.gzmB
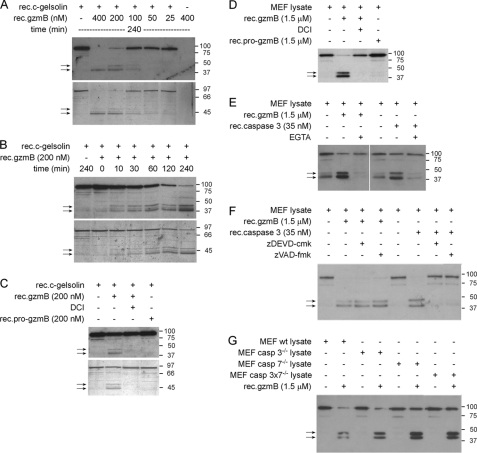
The original finding that c-gelsolin is cleaved by caspase 3 into 2 fragments with a calculated molecular mass of 39 kDa (NH2-terminal half) and 41 kDa (COOH-terminal half) during FasL/Fas-mediated apoptosis (11) was verified by treating MBL-2.Fas cells with anti-Fas mAb, Jo2. Engagement of Fas receptor led to activation of caspase 3 as analyzed by FACS (supplemental Fig. S1A) and Western blot (not shown), the generation of the expected two caspase 3-generated gelsolin fragments with an experimental molecular mass of ∼40 and ∼44 kDa, respectively (supplemental Fig. S1B), and induction of typical apoptotic markers, i.e. PS externalization and PI uptake (supplemental Fig. S1C). When incubated with increasing concentrations of rec. mouse gzmB (rec. gzmB) for 4 h, two main ∼40- and ∼44-kDa cleavage products of full-length recombinant mouse cytoplasmic gelsolin (rec.c-gelsolin; ∼90 kDa) were observed by Western blot (Fig. 1A, upper panel) and silver staining (Fig. 1A, lower panel). No degradation of untreated rec.c-gelsolin was observed (Fig. 1A), and the anti-gelsolin IgG did not cross-react with rec.gzmB (Fig. 1A). Rec.gzmB (200 nm) degraded rec.c-gelsolin in a time-dependent manner with the ∼40-and ∼44-kDa bands appearing after 10 min (Fig. 1B). Cleavage of rec.c-gelsolin was dependent on Ca2+ (supplemental Fig. S2A) and was blocked by the gzmB inhibitor DCI (Fig. 1C).
FIGURE 1.
Purified and lysate-associated c-gelsolin is cleaved by rec.gzmB independent of caspase 3. A and B, rec.c-gelsolin was treated with rec.gzmB in a dose (A)- and time-dependent manner (B). C, specific proteolysis of c-gelsolin by gzmB was verified using rec.gzmB, previously inactivated with DCI or the inactive form of gzmB (pro-gzmB) for 2 h. Cleavage patterns of gelsolin shown in A–C were analyzed by SDS-PAGE and Western blot using anti-nat. bovine plasma gelsolin IgG (upper panels) or by silver staining (lower panels). Arrows indicate main cleavage products. D, MEF wt lysate was incubated with rec.gzmB, rec.gzmB, previously inactivated with DCI, or with rec.pro-gzmB for 2 h. E, MEF wt lysate was incubated with rec.gzmB for 2 h or active rec.caspase 3 for 1 h in the absence or presence of 10 mm EGTA. F, MEF wt lysate or active rec.caspase 3 were preincubated with the caspase 3/7-specific inhibitor zDEVD-cmk or the pan-caspase inhibitor zVAD-fmk and subsequently incubated with rec.gzmB for 2 h or MEF wt lysate for 1 h, respectively. G, MEF wt, MEF casp3−/−, MEF casp7−/−, and MEF casp3 × 7−/− lysates were incubated with rec.gzmB for 2 h. Cleavage patterns of gelsolin shown in D–G were analyzed by Western blot using anti-rec.c-gelsolin IS. Arrows indicate cleavage products.
In control experiments the susceptibility of rec.c-gelsolin to cleavage by rec.caspase 3 was analyzed as described before (11) with the following results; there was a time-dependent appearance of two rec.c-gelsolin fragments of ∼40 kDa (NH2-terminal half) and ∼44 kDa (COOH-terminal half) (supplemental Fig. S2B); cleavage was independent of Ca2+ (supplemental Fig. S2A) and was inhibited by zDEVD-cmk (supplemental Fig. S2C). Under the given conditions, rec.c-gelsolin cleavage products were readily observed after 5 min with almost complete disappearance of the full-length protein after 30 min. The data suggest a higher catalytic efficiency for rec.caspase 3 versus rec.gzmB.
To examine susceptibility of native c-gelsolin to proteolysis by rec.gzmB, cell lysates from MEF wt (Fig. 1D) and EL4 cells (supplemental Fig. S2D) were incubated with the protease (1.5 μm) for 2 h, and lysates were immunoblotted with anti-gelsolin IS. C-gelsolin was processed again, yielding ∼40- and ∼44-kDa bands (Fig. 1D) similar to fragments obtained with rec.caspase-3 (Fig. 1E). Cleavage of c-gelsolin by rec.gzmB and rec.caspase 3 was dependent on Ca2+ (Fig. 1E) with proteolysis blocked, respectively, by DCI and zDEVD-cmk/zVAD-fmk (Fig. 1, D and F). Furthermore, to verify that gzmB-mediated cleavage of c-gelsolin was not due to previously activated caspase 3 (7), rec.gzmB was added to MEF lysate conditioned with zDEVD-cmk/zVAD-fmk (Fig. 1F) or to lysates from MEF cells that lacked either caspase 3 or caspase 7 or both proteases (Fig. 1G). C-gelsolin remained susceptible to cleavage by rec. gzmB under these conditions, suggesting that gzmB indeed directly processes c-gelsolin in the context of cytoplasmic proteins.
Rec.gzmB Cleaves c-gelsolin Mainly at Asp352
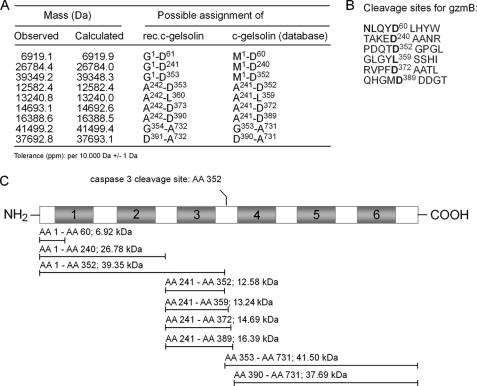
LC/ES-MS was performed to determine the cleavage site(s) of rec.c-gelsolin by rec.gzmB. For this purpose, rec.c-gelsolin, preincubated with rec.gzmB in the presence or absence of DCI as well as samples of rec.c-gelsolin and rec.gzmB alone, were analyzed simultaneously by Western blot to verify proper cleavage (supplemental Fig. S3A) and by LC/ES-MS (supplemental Fig. S3B). The amino acid (AA) sequence of rec.c-gelsolin differs from the published AA sequence of c-gelsolin available in Swissprot. rec.c-gelsolin, due to the cloning strategy, starts with the GSVVEH, whereas the published sequence is MVVEH (29). The mass of each fragment was calculated on the basis of the AA sequence of rec. c-gelsolin, but the numbering in the figures refers to the published AA sequence. The overlay of the total ion current chromatogram of rec.mgzmB (red) and rec.c-gelsolin (green) (supplemental Fig. S3B, upper panel) shows that the preparation of rec. c-gelsolin contains in addition to the intact rec.c-gelsolin eluting at 30.99 min several breakdown products (27.52, 29.15, 29.82, 30.35, 32.36 min) of the protein that are also observed by Western blot (supplemental Fig. S3A).
Incubation of rec.c-gelsolin with rec.gzmB (supplemental Fig. S3B, lower panel) led to the reduction of the main gelsolin peak (arrow) and the generation of new signals, the most prominent eluting around 27.5 and 31.5 min (rectangle). The analysis of the LC/ES-MS data revealed nine additional fragments with six cleavage sites (Fig. 2, A and B) that were not present in the LC/ES-MS data of rec.c-gelsolin alone. The averaged spectra of the identified fragments are depicted in supplemental Fig. S3C. One of the rec.gzmB-induced rec.c-gelsolin cleavage sites (Fig. 2C) is identical to that obtained with caspase 3, namely PDQTD352 (11). Together with the previous Western blot analyses, these data suggest that rec.gzmB and rec.caspase 3 mainly cleave rec.c-gelsolin at the same AA residue. The finding that five out of six cleavage sites of rec.gzmB are processed after aspartic acid, is consistent with the known specificity of mouse gzmB (12). Five of the fragments generated resulted from cleavage after the sequence TAKED240, which is located between domain 2 and 3 and, thus, might represent another (dominant) gzmB cleavage site within c-gelsolin. However, the sequence of cleavage events will require detailed kinetic analyses.
FIGURE 2.
Nine fragments of rec.c-gelsolin generated by rec.gzmB are identified by LC/ES-MS. A, observed and calculated/theoretical masses of the identified fragments of rec.c-gelsolin are listed as well as the possible assignments and flanking AA of the used rec.c-gelsolin or of the published AA sequence of mouse c-gelsolin. B, the six calculated gzmB cleavage sites of rec.c-gelsolin are listed (bold face). C, a schematic representation of the cleavage products of rec.c-gelsolin, observed by LC/ES-MS analysis, is depicted as lines in relation to the full-length gelsolin molecule. The NH2- and COOH-terminal AA of each fragment as well as the molecular mass of the fragment are shown. The previously determined cleavage site of caspase 3 is depicted above the diagram.
GzmB-generated Cleavage Fragments of c-gelsolin Depolymerize Actin Filaments in Vitro
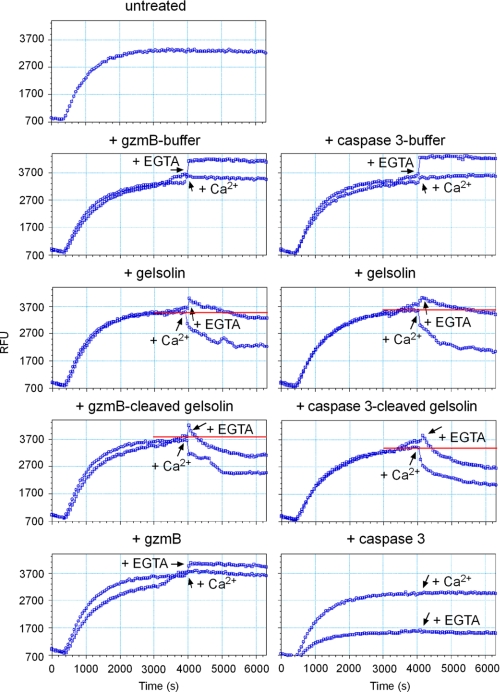
To determine the potential significance of rec.c-gelsolin cleavage by rec.gzmB, the activity of full-length c-gelsolin and gzmB-generated fragments were evaluated by an in vitro actin depolymerization assay (11). Globular actin monomers labeled with pyrene are polymerized into filamentous actin (F-actin) by adding MgCl2, KCl, and ATP. The conversion of globular actin into F-actin is accompanied by a greater than 5-fold increase in fluorescence. Depolymerization of F-actin by full-length rec.c-gelsolin is strictly dependent on Ca2+ (Fig. 3). However, rec.caspase 3 or rec. gzmB-cleaved rec.c-gelsolin severs F-actin in the absence of Ca2+ (Fig. 3). This dichotomy is likely explained by the requirement for Ca2+ to bind to the COOH-terminal portion of rec.c-gelsolin to uncover, through a change in topology, the severing capacity of the NH2-terminal c-gelsolin fragment (30–32). The appropriate buffers or proteases alone did not influence the polymerized status of F-actin (Fig. 3).
FIGURE 3.
GzmB-cleaved gelsolin is activated and severs actin filaments in a Ca2+-independent manner in vitro. Globular actin was polymerized into F-actin by the addition of KCl, CaCl2, and ATP. After reaching the plateau (polymerized F-actin), the appropriate buffer, intact rec.c-gelsolin, rec.gzmB- or rec.caspase3-cleaved rec.c-gelsolin, or the protease alone was added in the presence or absence of 10 mm EGTA, or F-actin was left untreated. The fluorescence was measured every minute (excitation, 350 nm; emission, 407 nm). Each blue dot indicates a time point of measurement. RFU, relative fluorescence units.
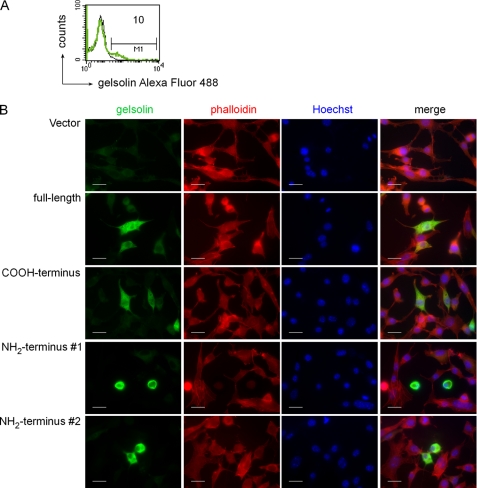
We then examined whether fragments of rec.c-gelsolin generated by rec.gzmB altered actin cytoskeleton morphology in intact cells. The c-gelsolin knock-out cell line (MC.Gelsolin−/−), generated from gelsolin knock-out mice (21) was transfected (Fig. 4) with cDNAs for either full-length rec.c-gelsolin or the NH2-terminal (1–353) or the COOH-terminal (354–731) fragments, corresponding to the cleavage products generated by gzmB and caspase 3. rec.c-gelsolin, actin, and nuclei of the transfected cells (10% efficiency; Fig. 4A) were stained, respectively, with anti-gelsolin IS, phalloidin (actin binding fungal toxin), and Hoechst 33342. Transfection with the full-length c-gelsolin or the COOH-terminal fragment of c-gelsolin led to expression of c-gelsolin (green), which was evenly distributed throughout the cytoplasm (Fig. 4B). The cell morphology appeared normal, and the actin filaments were readily visible. In contrast, upon expression of the NH2-terminal fragment of c-gelsolin, which is predicted to be constitutively active (11), MC.Gelsolin−/− cells exhibited significant morphological alterations, namely rounding up of cells (Fig. 4B). However, no apparent signs of apoptosis, such as the generation of apoptotic bodies or nuclear fragmentation, investigated by fluorescence microscopy were observed under these conditions. The observation that the cells productively transfected with the NH2-terminal fragment of gelsolin do not undergo programmed cell death despite their altered morphology is further supported by data showing the absence of DNA fragmentation by the TUNEL assay (supplemental Fig. S4).
FIGURE 4.
Transfection of MC.Gelsolin−/− cells with the NH2-terminal half of gelsolin induces changes in cell morphology. MC.Gelsolin−/− cells were transfected with a control vector or with vectors coding for full-length gelsolin and the COOH- or NH2-terminal half of rec.c-gelsolin. A, the efficiency of transfection was determined by intracellular FACS-staining of MC.Gelsolin−/− cells transfected with the control vector (black line) or with vector coding for full-length gelsolin (green line) using anti-rec.c-gelsolin IS. The number corresponds to the percentage of cells, which are positive for gelsolin. B, transfected cells were stained with anti-rec.c-gelsolin IS and rhodamine phalloidin Alexa Fluor 546 for fluorescence microscopy and mounted in a drop of Fluoromount G containing 10 μg/ml Hoechst 33342. Representative microscopic images were taken from corresponding slides at room temperature using a Zeiss microscope (Imager Z1), a Zeiss Axiocam as analysis camera, and Zeiss Vision as software. The objective used was a Zeiss Plan-Apochromat. #1 and #2 indicate different areas on the coverslip. Scale bars, 20 μm.
GzmB of ex Vivo LCMV-immune Tc Cells Induces Cleavage of c-gelsolin in MEF wt Cells via Activation of Caspases
Rec. gzmB, delivered by SLO, cleaves c-gelsolin in intact cells producing the ∼40- and 44-kDa fragments (Fig. 5A). Similar results were obtained when gp33-pulsed MEF wt cells were incubated with the combination of soluble rec.gzmB and ex vivo-derived virus-immune Tc cells that expressed perf but neither gzmA nor gzmB (gzmAxB−/− Tc) as the delivery agent (33) (supplemental Fig. S5A). In addition to c-gelsolin cleavage, intracellular delivery of rec.mgzmB by SLO was accompanied by activation of caspase 3 supplemental Fig. S5B, upper panel) as well as PS exposure and PI incorporation (supplemental Fig. S5B, lower panel). However, in contrast to the data obtained with cell lysates, c-gelsolin cleavage after intracellular delivery of rec.gzmB via SLO was inhibited to nearly background level by zDEVD-cmk and zVAD-fmk (Fig. 5A), indicating that activation of caspases, including caspase 3, in intact cells is necessary for processing c-gelsolin.
FIGURE 5.
The cleavage of c-gelsolin in intact cells by purified or Tc cell-associated gzmB is dependent on caspase 3. A, MEF wt cells preincubated with the caspase 3/7-specific inhibitor zDEVD-cmk or the pan-caspase inhibitor zVAD-fmk were treated with rec.gzmB ± 0.25 μg/ml SLO for 2 h. Cleavage patterns of gelsolin were analyzed in cell lysates of MEF wt cells by Western blot using anti-rec.c-gelsolin IS. Arrows indicate cleavage products of gelsolin. B, Gp33-pulsed or untreated MEF wt cells were incubated with ex vivo LCMV-immune B6 Tc cells (effector to target ratio 20:1) for the indicated time points. C, Gp33-pulsed or untreated MEF wt or MEF casp3 × 7−/− cells were incubated with ex vivo LCMV-immune gzmB+ Tc cells (effector to target ratio 20:1) for the indicated time points. D, Gp33-pulsed or untreated MEF wt cells were incubated with ex vivo LCMV-immune gzmB+ Tc cells (effector to target ratio 20:1) for 1 h in the presence or absence of the caspase3/7-specific inhibitor zDEVD-cmk or the pan-caspase inhibitor zVAD-fmk. Cleavage patterns of gelsolin shown in B–D were analyzed in cell lysates by Western blot using anti-rec.c-gelsolin IS. Arrows indicate cleavage products of gelsolin. PS exposure and PI incorporation were analyzed by FACS staining in the cell population negative for CD8 expression. Numbers correspond to the percentage of cells in each quadrant. FITC, fluorescein isothiocyanate.
To further evaluate whether gzmB is able to directly cleave c-gelsolin when delivered under physiological conditions, gp33-pulsed or untreated MEF wt cells were challenged with ex vivo LCMV-immune CD8+ wt or gzmB+ (gzmA−/−) Tc cells. As control, virus-immune CD8+ Tc cells deficient in either gzmB (gzmA+ Tc) or both gzmA and gzmB (gzmAxB−/− Tc) were employed. All four Tc populations consisted of 80–90% CD8+ cells, 8–10% of which were reactive toward gp33 (supplemental Fig. S6A, only shown for wt Tc cells and (7)). The transcripts for perf, gzmA, and/or gzmB (supplemental Fig. S6B) and the respective proteins (supplemental Fig. S6C; only shown for gzmA and gzmB) were comparable for the four Tc populations. Furthermore, all effector populations expressed similar amounts of gzmK mRNA, low levels of gzmC mRNA, but no transcripts for gzms D-G (supplemental Fig. S6B).
When incubated with ex vivo LCMV-immune B6 (Fig. 5B) or gzmB+ (Fig. 5, C and D) Tc cells for 1 h at an effector to target ratio of 20:1, 2 c-gelsolin fragments with the MW of ∼40 and ∼44 kDa were observed in gp33-pulsed but only marginally, if at all, in untreated MEF wt cells. Tc cells alone only produced a marginal signal for c-gelsolin even when applied at a 20-fold excess (Fig. 5B, first lane; shown for B6 only) indicating that full-length c-gelsolin and its fragments seen in the Western blot were mainly derived from MEF cells. This finding is likely due to the smaller cytoplasmic volumes of a Tc cell compared with a MEF cell. The intensity of the two c-gelsolin fragments induced by B6 Tc cells increased after 2 h with a decline of the full-length band thereafter (Fig. 5B). The signal for both full-length c-gelsolin and the two fragments disappeared at 4 h, suggesting loss of target cells and consequently of protein due to cell death. This assumption is supported by the FACS data showing about 40% of PS+ and PI+ target cells (Fig. 5B). Specific cleavage of c-gelsolin by B6 or gzmB+ Tc cells was accompanied by a time-dependent increase in PS exposure and PI incorporation for the gp33-pulsed but not untreated MEF wt cells (Fig. 5, B and C). A similar induction of c-gelsolin cleavage and pro-apoptotic markers by B6 and gzmB+ Tc cells was also observed in EL4.F15 (supplemental Fig. S7A) and in RMA cells (supplemental Fig. S7B). In contrast, no or only marginal proteolysis of c-gelsolin and no induction of pro-apoptotic signals (PS exposure) were seen with gzmA+ (gzmB−/−) or gzmAxB−/− Tc cells under these conditions (supplemental Fig. S7, C and D, and see below) excluding the involvement of gzmA, gzmK, and/or gzmC in these processes.
Because MEF wt cells were found to express Fas (supplemental Fig. S8A), it was possible that c-gelsolin cleavage was elicited by FasL/Fas interaction and subsequent activation of caspase 3. However, ex vivo LCMV-immune Tc cells, which expressed a defective mutant of FasL but normal levels of perf and gzms (gld/gld) (34), induced c-gelsolin fragmentation and PS externalization in gp33-pulsed MEF wt cells at levels similar to B6 Tc cells (supplemental Fig. S8B). In contrast, when the same target cells were incubated with ex vivo LCMV-immune perfxgzmAxB−/− Tc cells, which express FasL but neither perf nor gzmA/gzmB, no c-gelsolin cleavage and PS exposure was observed. The functionality of FasL expressed by perfxgzmAxB−/− Tc cells was verified by their potential to induce c-gelsolin fragmentation and PS exposure in gp33-pulsed MBL-2.Fas but not the MEF wt cells (supplemental Fig. S8C).
Although the data indicate that gzmB is the essential for initiating the induction of c-gelsolin cleavage by the Tc cell granule secretory pathway, neither c-gelsolin cleavage nor signs of apoptosis were observed in caspase 3/caspase 7-deficient MEFs exposed to gzmB+ virus-immune Tc cells (Fig. 5C). Similar results were noted when MEF wt cells were pretreated with the caspase inhibitors zDEVD-cmk or zVAD-fmk (Fig. 5D). Therefore, the data support the model in which gzmB secreted by a cytotoxic cell initiates a pathway of gelsolin proteolysis that is executed by caspase 3 and 7.
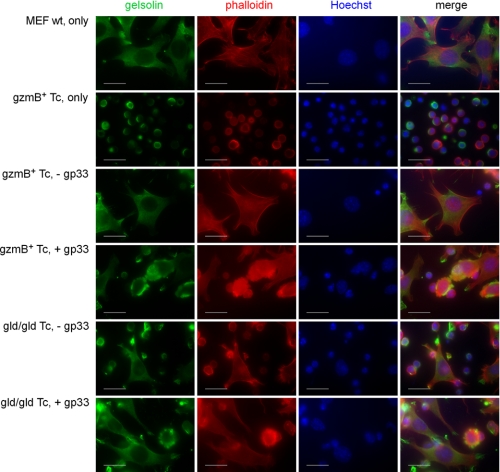
GzmB Secreted by Tc Cells Initiates c-gelsolin Cleavage in MEF wt Cells That Alters Cell Morphology
To test whether c-gelsolin cleavage by Tc cell-associated gzmB is accompanied by changes in target cell morphology, gp33-pulsed or untreated MEF wt cells were incubated with ex vivo LCMV-immune gzmB+ or gld/gld Tc cells for 1 h followed by staining with anti-rec.c-gelsolin IS (gelsolin), actin binding toxin phalloidin (actin), and Hoechst (nucleus) (Fig. 6). MEF wt cells alone show an even cytoplasmic distribution of c-gelsolin in the cytoplasm and a normal F-actin morphology. However, as expected from resting cells, c-gelsolin and actin do not colocalize (see Fig. 6, merge). Tc cells alone also stained for c-gelsolin and actin, but their morphology is readily distinguishable from the MEF wt cells. After incubation with gzmB+ or gld/gld Tc cells, typical apoptotic morphology was seen in gp33-pulsed but not mock-treated MEF wt cells. Gelsolin accumulated in apoptotic bodies, and the actin staining localized around the nucleus, suggesting a breakdown of F-actin into shorter fragments.
FIGURE 6.
GzmB+ and gld/gld ex vivo Tc cells induce apoptotic features in MEF wt target cells. Gp33-pulsed or untreated MEF wt cells were incubated with ex vivo LCMV immune gzmB+ or gld/gld Tc cells (effector to target ratio 20:1) for 1 h. The cells were stained with anti-rec.c-gelsolin IS and rhodamine phalloidin Alexa Fluor 546 for fluorescence microscopy and mounted in a drop of Fluoromount G containing 10 μg/ml Hoechst 33342. Representative microscopic images were taken from corresponding slides at room temperature using a Zeiss microscope (ImagerZ1), a Zeiss Axiocam as analysis camera, and Zeiss Vision as software. The objective used was a Zeiss Plan-Apochromat. Scale bars, 20 μm.
DISCUSSION
The present study shows that gzmB cleaves both the isolated and cell lysate-associated forms of c-gelsolin in vitro but that proteolysis mediated by gzmB secreted by ex vivo immune Tc cells requires caspases 3 and/or 7. The NH2-terminal c-gelsolin fragments generated via gzmB disrupt actin filaments in vitro independently of Ca2+, as shown before for caspase 3 (11). Moreover, gzmB of Tc cells induces disintegration of the actin cytoskeleton in target cells. In light of the fact that certain viruses need an intact cytoskeleton for infection of cells, duplication, and/or egress (35–37), one could speculate that Tc cells interfere with viral life cycle through this novel gzmB-induced intracellular proteolytic pathway.
Numerous substrates have been reported for gzmB, usually based on the single criteria that isolated or cell extract-associated protein is cleaved by the protease (3, 5, 38, 39). As reported elsewhere (40, 41), additional criteria must be fulfilled before a protein is accepted as a biological relevant substrate. The requirements include 1) achieving a minimum catalytic efficiency for the observed proteolytic event that is further documented by mutating AA at the cleavage site and 2) showing that the protein is similarly cleaved after gzmB is delivered into intact cells by such agents as perf, SLO, and adenoviral particles. However, as reported here and suggested previously (5, 40), these two requirements should be complemented by experiments that examine proteolysis of the protein in target cells after exposure to ex vivo immune Tc cells that selectively secrete gzmB and have their caspase cascade paralyzed either by inhibitors or through knock out technology. Using these stringent criteria, we show here that although gzmB cleaves c-gelsolin with reasonable efficiency, the protein is not directly processed by this protease upon Tc cell delivery into MEF target cells, but gzmB instead initiates a caspase cascade that ultimately is responsible for proteolysis. We cannot exclude the possibility that gzmB directly processes c-gelsolin in specific subtypes of cells. For example, the B23 autoantigen is most susceptible to cleavage by gzmB in differentiated smooth muscle cells (42).
What factors contribute to the failure of gzmB to cleave c-gelsolin when delivered to target cells either by SLO or Tc cells? The failure of gzmB to cleave c-gelsolin in intact cells may be due to the relative inaccessibility of the protein coupled with the possible discrete localization of the protease in the cytosol after delivery by the Tc cell. Similar discrepancies have been reported for the capacity of gzmB to cleave α-tubulin and β-actin in YAC-1 cells. Here, gzmB cleaved the substrates in cell lysates in the presence of caspase inhibition but proteolysis in intact cells required caspase activation (38). A similar dichotomy has been observed in which gzmB processes caspase 3 but not the highly preferred caspase 7 in breast cancer cells (43). Furthermore, because gzmB+ Tc cell-mediated apoptosis activates caspase 3 (7, 44–47) either directly or through amplification via Bid (48), the level of active caspase 3 is predicted to progressively rise as the cell undergoes apoptosis, whereas the level of Tc-delivered gzmB remains limited and insufficient for c-gelsolin cleavage. This possibility is strengthened by the observation that the catalytic efficiency of gzmB toward rec.c-gelsolin in vitro is at least an order of magnitude less than caspase 3. Based on the data presented here, Tc cells have the capacity to induce cleavage of c-gelsolin independently via the FasL/Fas pathway (11, 49) and via granule exocytosis, suggesting that this proteolytic process is evolutionarily conserved and, thus, has substantial biological relevance. However, whether proteolysis of gelsolin contributes to the apoptotic phenotype remains uncertain. Recent studies have shown that caspase 3-processed c-gelsolin severs isolated actin filaments in a Ca2+-independent manner and causes depolymerization in permeabilized fibroblasts (11) and that the expression of the NH2-terminal c-gelsolin fragment in multiple cell types causes the cells to round up, detach from their substratum, and undergo nuclear fragmentation (11, 50). Why transfection of c-gelsolin-deficient cells with cDNAs encoding the fragments generated by caspase 3 or gzmB do not induce apoptotic morphology, as reported herein, is perplexing. Additional factors, such as differential level of expression of the NH2-terminal fragment of gelsolin or the quality of target cells employed, including other proteolytic events, may influence the response of a target cell to cleaved c-gelsolin after an encounter with Tc cells. However, the experimental protocol employed does not allow one to verify whether gzmB- and caspase 3-mediated c-gelsolin cleavage contributes to gzmB-facilitated apoptosis. A putative connection between these processes may only become evident when studied in cells in which all substrates downstream of gzmB/caspase 3 with pro-apoptotic potential are deleted or blocked.
As gelsolin cleavage appears to be dispensable for Tc cell-mediated cell death, what other biological processes might be modified? Tc cells are presumed to control viral replication and eliminate tumor cells via mechanisms that culminate in cell death (1). However, recent evidence suggests that gzms secreted by Tc cells possess non-cytotoxic activities that include such diverse biological effects as induction of pro-inflammatory cytokines, remodeling of extracellular matrices, and inactivation of intracellular pathogens (4–6, 51, 52). In this regard, gzmH has been reported to inactivate adenoviral proteins necessary for replication (53), and gzmB appears to block the activation of herpes simplex virus-1 in latently infected neurons (54). Certain viruses such as parvovirus, human immunodeficiency virus, human T cell leukemia virus, or herpes simplex virus depend on an intact actin cytoskeleton either for their entry, replication, and/or egress from infected cells (35–37). GzmB, therefore, might influence viral life cycle by initiating a proteolytic cascade that cleaves cytoskeletal components such as c-gelsolin. The data here, therefore, broaden the functionality of gzmB to include the disruption of survival strategies enacted by intracellular pathogens.
Supplementary Material
Acknowledgments
We thank Thomas Stehle and Anton Grubisic for expert technical assistance. Many thanks go to Sucharit Bhakdi for the generous gift of SLO. We also thank Martin Biniossek for preliminary experiments, critical comments, and stimulating discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant R01AI044941 (to C. J. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S8.
- Tc cell
- cytotoxic T lymphocyte
- AA
- amino acid
- c-gelsolin
- cytoplasmic gelsolin
- DCI
- dichloroisocoumarin
- FACS
- fluorescence-activated cell sorting
- F-actin
- filamentous actin
- gzm
- granzyme
- IS
- immune serum
- LC/ES-MS
- liquid chromatography/electrospray-mass spectrometry
- LCMV
- lymphocytic choriomeningitis virus
- mAb
- monoclonal antibody
- MEF
- mouse embryonic fibroblast
- nat.
- native
- perf
- perforin
- NK cell
- natural killer cell
- PI
- propidium iodide
- PS
- phosphatidylserine
- rec.
- recombinant
- SLO
- streptolysin O
- TUNEL
- deoxynucleotidyltransferase-mediated dUTP nick end labeling
- wt
- wild type
- zDEVD-cmk
- benzyloxycarbonyl-DEVD- chloromethyl ketone
- zVAD-fmk
- benzyloxycarbonyl-VAD-fluoromethyl ketone.
REFERENCES
- 1.Russell J. H., Ley T. J. (2002) Annu. Rev. Immunol. 20, 323–370 [DOI] [PubMed] [Google Scholar]
- 2.Kägi D., Ledermann B., Bürki K., Zinkernagel R. M., Hengartner H. (1996) Annu. Rev. Immunol. 14, 207–232 [DOI] [PubMed] [Google Scholar]
- 3.Chowdhury D., Lieberman J. (2008) Annu. Rev. Immunol. 26, 389–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardo J., Aguilo J. I., Anel A., Martin P., Joeckel L., Borner C., Wallich R., Müllbacher A., Froelich C. J., Simon M. M. (2009) Microbes Infect. 11, 452–459 [DOI] [PubMed] [Google Scholar]
- 5.Froelich C. J., Pardo J., Simon M. M. (2009) Trends Immunol. 30, 117–123 [DOI] [PubMed] [Google Scholar]
- 6.Metkar S. S., Menaa C., Pardo J., Wang B., Wallich R., Freudenberg M., Kim S., Raja S. M., Shi L., Simon M. M., Froelich C. J. (2008) Immunity 29, 720–733 [DOI] [PubMed] [Google Scholar]
- 7.Pardo J., Wallich R., Martin P., Urban C., Rongvaux A., Flavell R. A., Müllbacher A., Borner C., Simon M. M. (2008) Cell Death Differ. 15, 567–579 [DOI] [PubMed] [Google Scholar]
- 8.Adrain C., Duriez P. J., Brumatti G., Delivani P., Martin S. J. (2006) J. Biol. Chem. 281, 8118–8125 [DOI] [PubMed] [Google Scholar]
- 9.Goping I. S., Sawchuk T., Underhill D. A., Bleackley R. C. (2006) J. Cell Sci. 119, 858–865 [DOI] [PubMed] [Google Scholar]
- 10.Waterhouse N. J., Oliaro J., Pinkoski M. J. (2006) Cell Death Differ. 13, 1839–1841 [DOI] [PubMed] [Google Scholar]
- 11.Kothakota S., Azuma T., Reinhard C., Klippel A., Tang J., Chu K., McGarry T. J., Kirschner M. W., Koths K., Kwiatkowski D. J., Williams L. T. (1997) Science 278, 294–298 [DOI] [PubMed] [Google Scholar]
- 12.Harris J. L., Peterson E. P., Hudig D., Thornberry N. A., Craik C. S. (1998) J. Biol. Chem. 273, 27364–27373 [DOI] [PubMed] [Google Scholar]
- 13.Sun H. Q., Yamamoto M., Mejillano M., Yin H. L. (1999) J. Biol. Chem. 274, 33179–33182 [DOI] [PubMed] [Google Scholar]
- 14.Spinardi L., Witke W. (2007) Subcell. Biochem. 45, 55–69 [DOI] [PubMed] [Google Scholar]
- 15.Burtnick L. D., Koepf E. K., Grimes J., Jones E. Y., Stuart D. I., McLaughlin P. J., Robinson R. C. (1997) Cell 90, 661–670 [DOI] [PubMed] [Google Scholar]
- 16.De Corte V., Bruyneel E., Boucherie C., Mareel M., Vandekerckhove J., Gettemans J. (2002) EMBO J. 21, 6781–6790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li G. H., Shi Y., Chen Y., Sun M., Sader S., Maekawa Y., Arab S., Dawood F., Chen M., De Couto G., Liu Y., Fukuoka M., Yang S., Da Shi M., Kirshenbaum L. A., McCulloch C. A., Liu P. (2009) Circ. Res. 104, 896–904 [DOI] [PubMed] [Google Scholar]
- 18.Burtnick L. D., Urosev D., Irobi E., Narayan K., Robinson R. C. (2004) EMBO J. 23, 2713–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwiatkowski D. J. (1999) Curr. Opin. Cell Biol. 11, 103–108 [DOI] [PubMed] [Google Scholar]
- 20.Balkow S., Kersten A., Tran T. T., Stehle T., Grosse P., Museteanu C., Utermöhlen O., Pircher H., von Weizsäcker F., Wallich R., Müllbacher A., Simon M. M. (2001) J. Virol. 75, 8781–8791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witke W., Sharpe A. H., Hartwig J. H., Azuma T., Stossel T. P., Kwiatkowski D. J. (1995) Cell 81, 41–51 [DOI] [PubMed] [Google Scholar]
- 22.Lakhani S. A., Masud A., Kuida K., Porter G. A., Jr., Booth C. J., Mehal W. Z., Inayat I., Flavell R. A. (2006) Science 311, 847–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardo J., Wallich R., Ebnet K., Iden S., Zentgraf H., Martin P., Ekiciler A., Prins A., Müllbacher A., Huber M., Simon M. M. (2007) Cell Death Differ. 14, 1768–1779 [DOI] [PubMed] [Google Scholar]
- 24.Ljunggren H. G., Kärre K. (1986) J Immunogenet 13, 141–151 [DOI] [PubMed] [Google Scholar]
- 25.van den Broek M. E., Kägi D., Ossendorp F., Toes R., Vamvakas S., Lutz W. K., Melief C. J., Zinkernagel R. M., Hengartner H. (1996) J. Exp. Med. 184, 1781–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhakdi S., Tranum-Jensen J., Sziegoleit A. (1985) Infect. Immun. 47, 52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin P., Wallich R., Pardo J., Müllbacher A., Munder M., Modolell M., Simon M. M. (2005) Blood 106, 2871–2878 [DOI] [PubMed] [Google Scholar]
- 28.Revell P. A., Grossman W. J., Thomas D. A., Cao X., Behl R., Ratner J. A., Lu Z. H., Ley T. J. (2005) J. Immunol. 174, 2124–2131 [DOI] [PubMed] [Google Scholar]
- 29.Dieffenbach C. W., SenGupta D. N., Krause D., Sawzak D., Silverman R. H. (1989) J. Biol. Chem. 264, 13281–13288 [PubMed] [Google Scholar]
- 30.Khaitlina S., Hinssen H. (2002) FEBS Lett. 521, 14–18 [DOI] [PubMed] [Google Scholar]
- 31.Lin K. M., Mejillano M., Yin H. L. (2000) J. Biol. Chem. 275, 27746–27752 [DOI] [PubMed] [Google Scholar]
- 32.Choe H., Burtnick L. D., Mejillano M., Yin H. L., Robinson R. C., Choe S. (2002) J. Mol. Biol. 324, 691–702 [DOI] [PubMed] [Google Scholar]
- 33.Kurschus F. C., Fellows E., Stegmann E., Jenne D. E. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13799–13804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi T., Tanaka M., Brannan C. I., Jenkins N. A., Copeland N. G., Suda T., Nagata S. (1994) Cell 76, 969–976 [DOI] [PubMed] [Google Scholar]
- 35.Bär S., Daeffler L., Rommelaere J., Nüesch J. P. (2008) PLoS Pathog. 4, e1000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fackler O. T., Kräusslich H. G. (2006) Curr. Opin. Microbiol. 9, 409–415 [DOI] [PubMed] [Google Scholar]
- 37.Clement C., Tiwari V., Scanlan P. M., Valyi-Nagy T., Yue B. Y., Shukla D. (2006) J. Cell Biol. 174, 1009–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bredemeyer A. J., Lewis R. M., Malone J. P., Davis A. E., Gross J., Townsend R. R., Ley T. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11785–11790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cullen S. P., Adrain C., Lüthi A. U., Duriez P. J., Martin S. J. (2007) J. Cell Biol. 176, 435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Damme P., Maurer-Stroh S., Plasman K., Van Durme J., Colaert N., Timmerman E., De Bock P. J., Goethals M., Rousseau F., Schymkowitz J., Vandekerckhove J., Gevaert K. (2009) Mol. Cell. Proteomics 8, 258–272 [DOI] [PubMed] [Google Scholar]
- 41.Froelich C. J., Metkar S. S., Raja S. M. (2004) Cell Death Differ. 11, 369–371 [DOI] [PubMed] [Google Scholar]
- 42.Ulanet D. B., Flavahan N. A., Casciola-Rosen L., Rosen A. (2004) Arthritis Rheum. 50, 233–241 [DOI] [PubMed] [Google Scholar]
- 43.Yang X., Stennicke H. R., Wang B., Green D. R., Jänicke R. U., Srinivasan A., Seth P., Salvesen G. S., Froelich C. J. (1998) J. Biol. Chem. 273, 34278–34283 [DOI] [PubMed] [Google Scholar]
- 44.Darmon A. J., Nicholson D. W., Bleackley R. C. (1995) Nature 377, 446–448 [DOI] [PubMed] [Google Scholar]
- 45.Lord S. J., Rajotte R. V., Korbutt G. S., Bleackley R. C. (2003) Immunol. Rev. 193, 31–38 [DOI] [PubMed] [Google Scholar]
- 46.Pardo J., Bosque A., Brehm R., Wallich R., Naval J., Müllbacher A., Anel A., Simon M. M. (2004) J. Cell Biol. 167, 457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quan L. T., Tewari M., O'Rourke K., Dixit V., Snipas S. J., Poirier G. G., Ray C., Pickup D. J., Salvesen G. S. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 1972–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metkar S. S., Wang B., Ebbs M. L., Kim J. H., Lee Y. J., Raja S. M., Froelich C. J. (2003) J. Cell Biol. 160, 875–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stegh A. H., Herrmann H., Lampel S., Weisenberger D., Andrä K., Seper M., Wiche G., Krammer P. H., Peter M. E. (2000) Mol. Cell. Biol. 20, 5665–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geng Y. J., Azuma T., Tang J. X., Hartwig J. H., Muszynski M., Wu Q., Libby P., Kwiatkowski D. J. (1998) Eur. J. Cell Biol. 77, 294–302 [DOI] [PubMed] [Google Scholar]
- 51.Romero V., Andrade F. (2008) Tissue Antigens 71, 409–416 [DOI] [PubMed] [Google Scholar]
- 52.Trapani J. A., Bird P. I. (2008) Immunity 29, 665–667 [DOI] [PubMed] [Google Scholar]
- 53.Andrade F., Fellows E., Jenne D. E., Rosen A., Young C. S. (2007) EMBO J. 26, 2148–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knickelbein J. E., Khanna K. M., Yee M. B., Baty C. J., Kinchington P. R., Hendricks R. L. (2008) Science 322, 268–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.