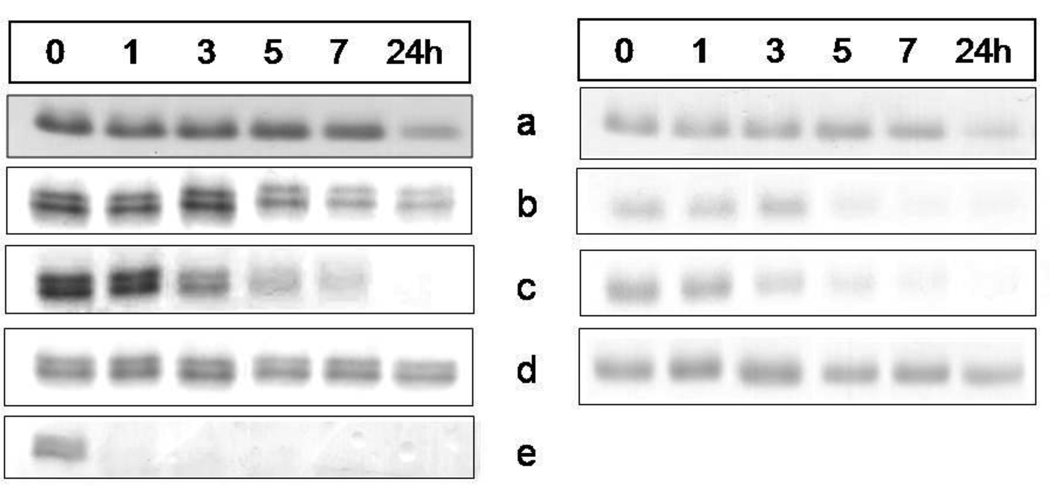
Figure 5.
SDS-PAGE showing the effect of interpain A on methaemoglobin species and haem-free globin chains
Hydroxy-methaemoglobin, pH 7.5, (a); aquomethaemoglobin, pH 6.0, (b), and pH 5.5 (c); azido-methaemoglobin, pH 6.0, (d); haem-free globin, pH 7.5, (e). Haemoglobin substrates at 4µM (with respect to tetramer) were incubated with InpA (2µM) at 37°C and aliquots withdrawn at indicated time points were subjected to the SDS-PAGE analysis. The hydroxy-methaemoglobin and aquomethaemoglobin species were formed by auto-oxidation of oxyhaemoglobin at 37°C for 24h at pHs 7.5, 6.0 and 5.5, and constituted 95% of the total haemoglobin present. Panel A, gels stained with coomassie blue; panel B, TMB-stained for haem-associated peroxidise activity. Azido-methaemoglobin was prepared by incubating aquomethaemoglobin in the presence of 400µM NaN3.