Abstract
TNFR/TNF superfamily members can control diverse aspects of immune function. Research over the past 10 years has shown that one of the most important and prominent interactions in this family is that between OX40 (CD134) and its partner OX40L (CD252). These molecules strongly regulate conventional CD4 and CD8 T cells, and more recent data are highlighting their ability to modulate NKT cell and NK cell function as well as to mediate cross-talk with professional antigen-presenting cells and diverse cell types such as mast cells, smooth muscle cells, and endothelial cells. Additionally, OX40-OX40L interactions alter the differentiation and activity of regulatory T cells. Blocking OX40L has produced strong therapeutic effects in multiple animal models of autoimmune and inflammatory disease, and, in line with a prospective clinical future, reagents that stimulate OX40 signaling are showing promise as adjuvants for vaccination as well as for treatment of cancer.
Keywords: OX40L, costimulation, T cells, autoimmunity, infectious disease, vaccination
INTRODUCTION
OX40 (also known as ACT35, CD134, TNFRSF4) was first discovered in 1987 with an antibody (termed MRC OX40) that bound to activated rat CD4 T cells (1). OX40 is an approximately 50-kD glycoprotein and has been cloned in the rat, mouse, and human (2–4). It is a type 1 transmembrane protein of 249 amino acids, with a 49 amino acid cytoplasmic tail and a 186 amino acid extracellular region. It was initially found to have homology to NGFR (nerve growth factor receptor) and CD40 and was then classified as a member of the TNFR (tumor necrosis factor receptor) superfamily, as more proteins with sequence and structural similarity to TNFR were discovered. The gene for OX40 is clustered on human chromosome 1 (mouse chromosome 4) with several other TNFR family molecules, TNFR2, 4-1BB, HVEM, CD30, GITR, and DR3. OX40 has three complete and one truncated cysteine-rich domains that are characteristic of the TNFR superfamily.
OX40’s ligand (OX40L, also known as gp34, CD252, TNFSF4) is a type II glycoprotein with a 23 amino acid cytoplasmic tail and a 133 amino acid extracellular domain. It was originally identified in 1985 on transformed T cell lines as a molecule, gp34, induced by the tax gene of human T cell leukemia virus (HTLV)-1 (5, 6), and then cloning of a binding partner for OX40 identified the protein gp34 as OX40L (7). It is expressed as a trimer and has a TNF homology domain; thus, it is structurally similar to other molecules of the TNF superfamily and has some sequence homology. The OX40L gene is on human and mouse chromosome 1, clustered with genes for two other TNF family members, FasL and GITRL. Although a number of the TNF family can bind to several partners, at present there is no evidence that OX40L can complex with anything other than OX40. Similarly, except for reports showing that feline OX40 can be a receptor for feline immunodeficiency virus (8), there are no indications of other binding partners for OX40 in human or mouse systems, including binding to other viruses. The crystallized complex of human OX40 and OX40L is a trimeric configuration of one OX40L molecule and three OX40 monomers (9). The length of the complex of the extracellular regions of OX40 bound to OX40L is approximately 80 Å, which when factored with a linker that connects to the transmembrane portion of OX40 makes the pairing compatible with bridging regions of 100–150 Å thought to exist between a number of interacting cell types, including T cells and antigen-presenting cells (APCs). This is similar to the sizes of other complexes such as those between the T cell receptor (TCR) and major histocompatibility complex (MHC) or CD28 and B7 that regulate T cell and APC activity and corresponds well with the functional data described below indicating essential costimulatory roles for the OX40-OX40L interaction in many aspects of immunity involving direct cell-cell communication.
OX40 AND OX40L EXPRESSION
The primary cell that is quoted most often as expressing OX40 is the activated T cell, and in fact some older literature stated that OX40 was T cell specific or restricted to CD4 T cells and on occasion restricted to Th2 cells. OX40 is likely predominantly expressed on activated T cells, but this includes CD4 and CD8 T cells, Th2, Th1, and Th17 cells, as well as Foxp3+CD4+ regulatory T cells (Tregs). Naive CD4 and CD8 T cells do not express OX40, nor do most memory T cells, whether central or effector memory phenotype. However, OX40 is induced with delayed kinetics after activation of naive T cells, with no real consensus in terms of timing, having been visualized anywhere from 12 h after activation to as long as 5–6 days. Memory T cells reexpress OX40 rapidly, within 1–4 h after activation. Differences between CD4 and CD8 T cells have been seen in some inflammatory situations, with OX40 expression appearing to be more transient on CD8 T cells, but it can be prolonged on CD4 T cells (10–13). Altered kinetics most likely relate to the nature of the antigen and its persistence, as well as to the inflammatory environment that accompanies antigen presentation, leading to the statement that the time and length of OX40 expression on conventional T cells cannot be generalized. Minimally, strong crosslinking of the TCR/CD3 complex can promote OX40 on CD4 and CD8 T cells, but under physiological conditions other costimulatory molecules such as CD28 contribute to the kinetics of appearance, and the cytokines IL-1, IL-2, IL-4, and TNF can enhance or prolong expression. OX40 is also found on Tregs. It is constitutively expressed on mouse natural (thymus-derived) Foxp3+ T cells, but it is only inducible on human Foxp3+ T cells. Other cell types can express OX40, although there is not a clear picture of when and where. These include natural killer T cells (NKT cells), neutrophils, and natural killer (NK) cells. The current ideas are that this is also induced expression, but data are not extensive at present. As described below, literature exists on the functional activity of OX40 on all of these cell types, implying that its effects are far broader than simply positively regulating conventional T cell immunity, even though most studies relate to the latter.
The primary source of OX40L is likely to be a professional APC, and it can be induced on activated B cells (14, 15), mature conventional dendritic cells (cDCs) (16, 17), Langerhans cells (18), plasmacytoid DCs (pDCs) (19, 20), and macrophages (21). Various APC maturation factors can promote OX40L, including signals through CD40, membrane B cell receptor, and several Toll-like receptors (TLR2, 4, 9), as well as inflammatory cytokines such as TSLP (thymic stromal lymphopoietin) (22) and IL-18 (23). However, additional immune cell types such as NK cells (24) and mast cells (25, 26), as well as structural cells such as vascular endothelial cells (27) and smooth muscle cells (28), can express OX40L, induced by the inflammatory cytokines GM-CSF, IL-1, and TNF (mast cells, smooth muscle cells) or by receptors such as NKG2D (NK cells). Furthermore, OX40L is inducible on some conventional activated T cells (29, 30) and on a specialized cell type called an adult lymphoid tissue inducer (LTi) that is CD4+ but CD3− and maintains the integral organization of lymphoid tissue but that might also function as an APC in some instances (31, 32).
Both OX40 and OX40L have been visualized in situ in mouse models of disease as well as in samples from human patients. Many of these data are summarized in Table 1. References have been omitted owing to space limitations, although some can be found elsewhere (33). One can have the impression from reports of in vivo expression that OX40 and OX40L are quite restricted; however, most of these analyses have focused on a particular cell type without attempting to analyze other cells. Therefore, the expression of these molecules in inflamed and diseased tissue might be much broader than is reflected in Table 1. A number of studies have tried to determine if either molecule can be used as a marker of disease progression or severity, with varying conclusions. Certainly there is strong support that they will be found primarily during an ongoing immune response, but given the inducible and transient nature of their expression, likely dictated by multiple other inflammatory factors, it is questionable whether they will be useful as conventional clinical biomarkers. However, expression studies during the course of any disease will be extremely valuable based on their therapeutic potential. OX40L can be cleaved from the membrane of cells, likely after binding to OX40, as implied by studies of mice deficient in OX40 in which greater levels of OX40L have been noted (Croft lab, unpublished data), and therefore any analysis of OX40L in situ might have the potential to underestimate its presence. In contrast, assessing OX40 expression should not have this limitation. Moreover, OX40 could have future uses for monitoring and isolating antigen-specific human T cells in in vitro diagnostic or functional assays. OX40 has been visualized on a few presumed memory CD4 T cells in peripheral blood, often associated with CD25 expression (34), and, perhaps more significantly, OX40 is upregulated on human T cells stimulated with recall viral antigen, bacterial antigen, and autoantigen (35, 36), again sometimes coexpressed with CD25. This latter finding suggests that OX40, in combination with other markers, might be suitable for identifying antigen-specific human T cell populations from in vitro recall assays.
Table 1.
Reported in situ expression of OX40 and OX40L in human and animal inflammationa
| Disease/response | Host | Organ/tissue | Cell type | OX40 | OX40L |
|---|---|---|---|---|---|
| MS, EAE | Human | Blood | T cells | + | |
| Mouse | CNS/spinal cord | CD4 T cells | + | ||
| CNS/spinal cord | CD11b+ MΦ/microglia | + | |||
| Rat | CNS/spinal cord | CD4 T cells | + | ||
| RA, CIA, adjuvant arthritis | Human | Blood/synovial fluid | CD4 T cells | + | |
| Synovial tissue | ? | + | |||
| Mouse | Blood | CD4 T cells | + | ||
| Rat | CD4 T cells | + | |||
| IBD, colitis, CD | Human | Gut | Endothelium | + | |
| Lamina propria | T cells | + | |||
| Mouse | Peyer’s patches, lamina propria | CD4 T cells | + | ||
| Mesenteric LN | cDCs | + | |||
| Asthma | Human | Lung | Smooth muscle | + | |
| Mouse | Periaortic LN, lung | CD4 T cells | + | ||
| Periaortic LN, lung | cDCs | + | |||
| Lung | B cells, MΦ | + | |||
| SLE, lupus nephritis, HgCl2-induced autoimmunity | Human | Kidney | Lymphocytes | + | |
| Kidney | Capillary endothelium | + | |||
| Blood | ? | + | |||
| Mouse, rat | CD4 T cells | + | |||
| Diabetes | Human | Blood | CD4 T cells | + | |
| Mouse | Pancreas | CD4 and CD8 T cells | + | ||
| Pancreas | cDCs | + | |||
| Heart disease (myocarditis, atherosclerosis, acute coronary syndrome) | Human | Heart | Myocytes | + | |
| Heart | Lymphocytes | + | |||
| Blood | Monocytes | + | |||
| Platelets ? | + | ||||
| Mouse | Heart, aorta | T cells | + | ||
| Myocytes | + | ||||
| Endothelial cells | + | ||||
| Macrophages | + | ||||
| Myasthenia gravis | Human | Blood | CD4 T cells | + | |
| Psoriasis | Human | Skin | CD4 and CD8 T cells | + | |
| Capillary endothelium | + | ||||
| Bone marrow transplants, GVHD | Human | Blood | CD4 and CD8 T cells | + | |
| Mouse | Blood | CD4 and CD8 T cells | + | ||
| Rat | Blood | CD4 T cells | + | ||
| Allografts | Human | Skin | CD4 T cells | + | |
| Skin | Endothelial cells | + | |||
| Mouse | Bowel | ? | + | + | |
| Cardiac | CD4 T cells | + | |||
| Skin | CD4 T cells | + | |||
| Tumors | Human | TIL | CD4 T cells | + | |
| TDLN | CD4 T cells | + | |||
| Mouse | TIL | CD4 and CD8 T cells | + | ||
| TDLN | CD4 and CD8 T cells, NKT cells | + | |||
| TIL | pDCs | + | |||
| HIV | Human | Blood | CD4 T cells | + | |
| Vaccinia virus | Mouse | Spleen, LN | CD8 and CD4 T cells | + | |
| Influenza virus | Mouse | CD4 and CD8 T cells | + | ||
| Cryptococcus neoformans | Mouse | CD4 and CD8 T cells | + | ||
| cDCs | + | ||||
| Leishmania major | Mouse | LN | CD4 T cells | + | |
| LN | cDCs | + | |||
| Nippostrongylus brasiliensis | Mouse | Spleen | cDCs | + | |
Abbreviations: CD, Crohn’s disease; CIA, collagen-induced arthritis; cDCs/pDCs, conventional/plasmacytoid dendritic cells; EAE, experimental allergic encephalomyelitis; aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; HIV, human immunodeficiency virus; IBD, inflammatory bowel disease; LN, lymph node; MS, multiple sclerosis; MΦ, macrophage; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TDLN, tumor-draining lymph node; TIL, tumor-infiltrating lymphocytes.
FUNCTION OF OX40 ON CD4 T CELLS
One of the most recognized and accepted activities of OX40-OX40L interactions is to regulate the division and survival of conventional T cells, which has led to the often-used description of OX40 as a costimulatory receptor for T cells. Hints at this important function were provided upon the initial discovery of OX40 in rat and human systems, where enhanced in vitro proliferation of CD4 T cells was reported with agonist antibodies to OX40, or trans-stimulation with OX40L (1, 7, 37). Furthermore, interim reports identified OX40 on pathogenic CD4 T cells at the site of inflammation in a model of MS (EAE) and suggested that depletion of such cells could ameliorate disease (38, 39). However, it was only with the advent of more physiological in vitro systems, the development of OX40 and OX40L knockout animals, and in vivo use of neutralizing or agonist antibodies specific for OX40L or OX40, respectively, that the strong role of the interaction in controlling T cells was fully appreciated (10, 11, 15, 40–44). These studies directly showed either altered division and survival of CD4 T cells in the presence or absence of OX40 or OX40L or a substantial reduction in the accumulation of antigen-specific CD4 T cells in the knockout systems, as well as impaired cytokine production either in vitro or in vivo. Furthermore, the development of CD4 T cell memory was strongly impaired in the gene-deficient animals (11, 15), and initial reports were published showing that blocking the OX40-OX40L interaction prevented CD4 T cell responses and the development of inflammation in models of MS, colitis, RA, GVHD, and leishmaniasis (21, 45–48). Directly suggesting that this is at least in part a function of OX40 signaling, in vivo administration of agonist antibodies to OX40 produced a reciprocal effect with markedly enhanced numbers of primary effector and memory CD4 T cells generated when antigen was delivered in various adjuvants (11, 49).
Further supporting the idea that OX40 plays an essential role in many situations in which enhanced or deregulated T cell activity occurs, investigators showed that ligation of OX40 prevented and, more significantly, reversed a state of unresponsiveness in the CD4 T cell compartment in several experimental systems of tolerance (50). In line with this finding, transgenic mice in which OX40L was constitutively expressed by DCs (51) or T cells (52) were characterized by the accumulation of greatly increased numbers of effector-like CD4 T cells after injection of antigen, and in the latter by spontaneous development of interstitial pneumonia, inflammatory bowel disease, and autoantibodies, presumably as a consequence of environmental exposure to mucosal antigens and subsequent deregulation of T cells. Illustrating that this is a physiological activity of OX40 when expressed on responding CD4 T cells are data with gene-deficient TCR transgenic T cells. These data demonstrate that T cells lacking OX40 do not sustain division for long periods after antigen recognition and are unable to maintain high levels of anti-apoptotic proteins, resulting in poor long-term survival of effector T cells and poor memory development (53, 54). Moreover, blocking OX40-OX40L interactions during secondary immune responses of memory CD4 T cells, or the use of memory-like T cells that cannot express OX40, has demonstrated that these molecules also strongly regulate clonal expansion of T cells to recall antigen (55). Recent data suggest that it is the effector memory subset of CD4 T cells that is primarily regulated by OX40 (56), although more studies are needed in this area before concluding that central memory CD4 T cells do not use or require OX40 as a costimulatory receptor. Reports using human CD4 T cells cultured in vitro have supported the mouse studies, showing that OX40-OX40L interactions can again control division and survival (24, 25, 37, 57–59), and two recent preclinical studies in nonhuman primates, either stimulating OX40 or blocking OX40L, describe strong effects on the development of CD4 T cell responses (60, 61). Collectively, these studies demonstrate that OX40 and OX40L dictate the number of effector (protective or pathogenic) T cells that accumulate in primary and secondary responses, as well as determine the frequency of memory T cells that are generated.
OX40 SIGNALING IN CD4 T CELLS
Studies of the intracellular molecules engaged and targeted by OX40 have provided good rationale for the strong impact of OX40 in regulating CD4 T cells (Figure 1). Initial studies, largely in transient transfection systems in non-T cells, found that OX40 can bind several TNFR-associated factors, with TRAF2, 3, and 5 being the principal molecules that can be recruited to the cytoplasmic tail (62, 63) via a QEE motif that is present in other family members (64). TRAF2 and 3 have been characterized as adaptor molecules that can lead to activation of both the canonical (IKKβ-dependent) NF-κB1 pathway as well as the noncanonical (NIK/IKKα-dependent) NF-κB2 pathway. Related to the initial findings in transfection systems of NF-κB1 activity after crosslinking OX40 (62, 63) are data in physiological systems in which CD4 T cells respond to antigen. These data show that OX40 strongly contributes to the overall level of NF-κB1 activation in T cells (65). CD4 T cells that lack OX40 cannot maintain high levels of several antiapoptotic Bcl-2 family members including Bcl-2, Bcl-xL, and Bfl-1 (53), a finding that directly correlates with reduced NF-κB1 activity (65). Furthermore, reconstitution of NF-κB1 signaling in these T cells restored the expression of Bcl-2, Bcl-xL, and Bfl-1 and reversed the defect in expansion and accumulation of T cells following antigen encounter (65).
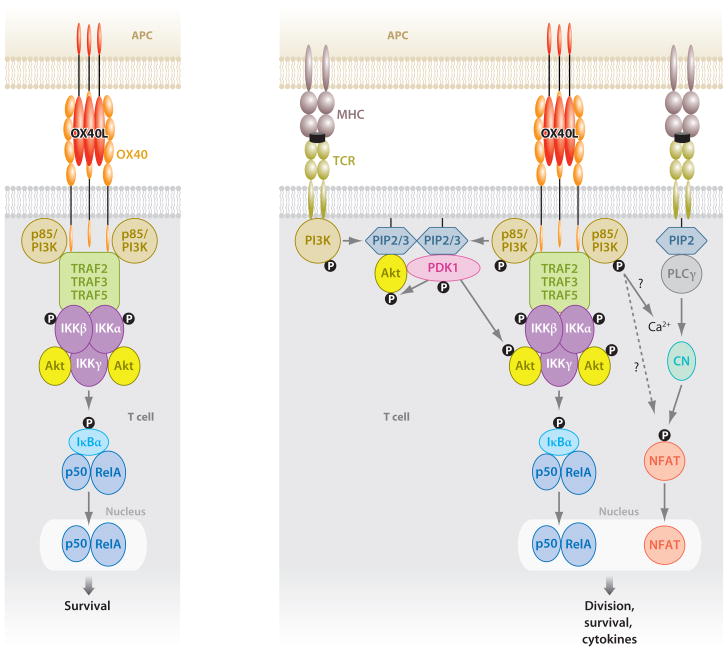
Figure 1.
OX40 acts as both an independent signaling entity (left) and an entity augmenting antigen-driven TCR signaling (right). Binding of OX40L results in trimerization of OX40 monomers and the recruitment of TRAF2, 3, and 5. The sequence of events resulting in a functional OX40 signaling complex is not yet clear, but IKKα, IKKβ, IKKγ, the p85 subunit of PI3K, and Akt are found with the TRAF adaptors. The signalosome can result in phosphorylation and degradation of IκBα, leading to activation of NF-κB1 and entry of p50 and RelA into the nucleus. This is sufficient to provide survival signals to T cells in the absence of antigen recognition. NIK is most likely also recruited into the complex, although this has not been visualized at present. If NIK is recruited, this likely leads to activation of NF-κB2, which might also be necessary for transmitting survival signals. In this scenario without antigen recognition, PI3K and Akt are not activated. With antigen signaling, OX40 synergizes with the TCR to promote strong Akt phosphorylation, likely due to localization of the OX40 signalosome with PDK1 recruited by the TCR. TCR signals initiate intracellular Ca2+ influx, leading to NFAT dephosphorylation and nuclear entry. Through a mechanism not yet elucidated, OX40 synergizes with this process to allow greater Ca2+ influx and nuclear accumulation of NFAT. The combined antigen and OX40 signals in T cells can enhance expression of molecules such as survivin, cyclin A, cyclin-dependent kinases, Bcl-2 antiapoptotic molecules, cytokines, and cytokine receptors, as well as suppress Foxp3 and CTLA4 expression. (Abbreviations: TRAF, TNFR-associated factor; PI3K, PI-3-kinase; NIK, NF-κB-inducing kinase; IKKs, IκB kinases; NFAT, nuclear factor of activated T cell; PDK1, 3-phosphoinositide-dependent kinase 1; CTLA4, cytotoxic T lymphocyte–associated antigen-4; PIP2/3, phosphatidylinositol 4,5-bisphosphate/3,4,5-trisphosphate; CN, calcineurin; PLCγ, phospholipase Cγ.)
OX40 signals also control the expression of survivin, a member of the IAP (inhibitor of apoptosis) family, that is induced in the G1 phase of the cell cycle and regulates G1-S transition and hence division of T cells (66). The finding that survivin also complexes with aurora B kinase and the mammalian target of rapamycin (mTOR) provided a link to earlier work demonstrating that OX40 also controls the phosphorylation of PI-3-kinase (PI3K) and Akt (protein kinase B), upstream activators of mTOR (54). This finding also connected this pathway directly with cyclin A and control of several cyclin-dependent kinases involved in cell cycle progression (67). Interestingly, decreased activity of Akt and NF-κB1 coincided in CD4 T cells that cannot receive OX40 signals, and forced expression of an active version of Akt restored defective expansion and survival of OX40-deficient T cells when responding to antigen (54), mimicking the action of an active version of IKKβ (65). This suggests that Akt and NF-κB1 cooperate to transmit the essential division and survival signals imparted by OX40.
Both published and unpublished studies have now provided a model of how OX40 acts as a costimulatory receptor (Figure 1). Upon ligation by OX40L expressed on APCs, OX40 is recruited into lipid rafts and forms a complex with TRAF2, 3, and 5, a complex that also contains IKKα, IKKβ, and IKKγ, as well as the p85 subunit of PI3K and Akt. OX40 can have two distinct modes of action. One is as a separate signaling unit, irrespective of antigen recognition and TCR signaling, that results in the phosphorylation of IKKα/β and subsequent nuclear accumulation of the subunits of NF-κB1, p50 and RelA. OX40 can therefore act as an independent receptor, much like TNF when binding to TNFR1/2, distinct from the classical version of a costimulatory receptor, which only functions in synergy with antigen signals through the TCR. The role of this independent activity appears to be principally to provide survival signals to T cells, although this needs to be investigated in more depth and may represent a way to sustain T cell responses after antigen is cleared. Sources of OX40L that could provide this antigen-independent signal might include LTi cells, B cells, and responding T cells themselves, which all can support T cell survival at the late effector phase of immune responses (30, 31, 68). When antigen is being presented, however, ligation of OX40 provides additional signals that are completely dependent on TCR signaling, one of which is focused on enhancing activation of the Akt pathway. This enhanced Akt activation will provide initial antigen-driven proliferative signals that further synergize with NF-κB1-driven growth and survival signals. Additionally, through an unknown mechanism, OX40 can also enhance TCR-induced calcium influx, leading to strong nuclear accumulation of NFATc1 and NFATc2 that likely regulate production of cytokines (69). TRAF2 is critical for the functional OX40 signaling complex in that it cannot form if TRAF2 is knocked down (T. So and M. Croft, unpublished observations), an observation that correlates with data demonstrating that CD4 T cells from a dominant-negative TRAF2 transgenic mouse were refractory to OX40 signals that drove generation of both effector and memory T cells (70).
OX40 can also result in activation of NIK and phosphorylation of IKKα, which leads to processing of p100 to p52 and hence to activation of noncanonical NF-κB2 (T. So and M. Croft, unpublished observation). The exact role played by TRAF3 and TRAF5 in the OX40 signaling complex is not clear. TRAF3 can complex with NIK and result in its degradation, meaning that NIK is not available for downstream effects. TRAF3 likely cooperates with TRAF2 in allowing OX40 to recruit NIK and then in allowing NIK to be released, remain intact, and become activated to drive the NF-κB2 pathway. What this controls in terms of functionality is not currently known, but NF-κB1 and NF-κB2 may synergize to fully provide division and survival signals through OX40.
CONTROL OF CYTOKINES AND T HELPER DIFFERENTIATION
Although direct intracellular signaling from OX40 can explain many of this receptor’s effects on regulating T cell division and survival, other indirect activities related to modulating cytokine production or cytokine receptor signaling further contribute to these processes as well as to the balance between CD4 T helper (Th) subsets that arise. OX40-OX40L interactions minimally impact initial IL-2 production from naive CD4 T cells, a phenomenon largely controlled by CD28 (10, 53), but OX40 signals can strongly promote IL-2 from effector T cells (10), as well as enhance expression of IL-2Rα that leads to gain of effector function typified by the ability to make IFN-γ upon exposure to cytokines such as IL-12 and IL-18 (71, 72). Other described activities that might contribute to the process of CD4 T cell differentiation include promoting IL-12Rβ2 expression (73) and blocking upregulation of CTLA4 (70), Foxp3 (74–76), and IL-10 (77). Given that CTLA4, Foxp3, and IL-10 are best characterized as suppressive molecules, inhibiting their expression would aid in driving a strong response of conventional CD4 T cells. Activation of Akt can block phosphorylation of Smad proteins that are required for TGF-βR to induce Foxp3, and therefore this might be one mechanism by which OX40 signals suppress Foxp3 expression (i.e., Treg generation). However, in one system, suppression of Foxp3 by OX40 was in part indirect, dependent on production of both IL-4 and IFN-γ (78), again implying that OX40-OX40L modulation of the cytokine environment can influence both the magnitude and the nature of the CD4 T cell response. How OX40 might antagonize IL-10 is not known, but this was illustrated in systems where vitamin D3 and dexamethasone were added into culture to induce Tregs that make IL-10 (77). This suggests either a novel action in blocking signaling through the receptors for these molecules or again an indirect effect related to production of Th1- or Th2-directive cytokines that could antagonize IL-10.
In naive CD4 T cells, under neutral conditions, OX40 engagement can preferentially lead to the generation of Th2 cells, driven by autocrine IL-4 and related to enhanced calcium/NFATc signaling, a phenotype again observed in both mouse and human systems (22, 40, 69, 79). However, the production of either IL-12 or IFN-α can overcome this Th2-directive action and result in the promotion, by OX40, of Th1 responses (19, 22, 80, 81). In one in vitro system, OX40 also antagonized the induction of Th17 cells, and this effect was mediated by autocrine IFN-γ (82). This action does not correlate with in vivo data showing that OX40 is necessary for EAE (21, 83) or in models of RA (48, 84), both Th17-controlled diseases. However, it again illustrates the ability of OX40-OX40L interactions to influence the immediate cytokine environment that might then influence Th differentiation. Certainly, ligation of OX40 can directly induce Th2, Th1, and Th17 cytokines in some situations (10, 23, 80, 85). Here the potential contribution of reverse signals through OX40L should be considered. Although much of the activity of OX40-OX40L interactions can be attributed to OX40 signaling, OX40L clearly can produce functional effects, as illustrated when crosslinked on cDCs and pDCs, B cells, and smooth muscle cells (14, 16, 28). This relates largely to production of cytokines such as IL-12, IL-6, IL-1, TNF, and IFN-α (16, 28, 86), several of which have strong activities in directing differentiation into various Th subsets (e.g., IL-12 or IFN-α for Th1; IL-6 for Th17). How OX40L transmits signals to promote proinflammatory cytokines is not known, but some studies have found alterations in PKCβ2, c-Jun, and c-Fos after OX40L is crosslinked (28, 87).
Thus, OX40-OX40L interactions have the ability to enhance an ongoing immune response regardless of the type of polarized response, both by imparting division and survival signals to differentiating or already differentiated T cells, by blocking the induction of Foxp3+ or IL-10+ Tregs, and by synergizing with and enhancing production of differentiative cytokines to additionally polarize the responses. This is clearly illustrated in disease studies in which the absence of OX40-OX40L interactions reduces clinical symptoms that are driven by Th1, Th2, and Th17 cells (Table 2).
Table 2.
Effect of manipulating OX40-OX40L interactions on inflammation and diseasea
| Inflammatory response | Host/treatment | Effect on disease or cellular immunity |
|---|---|---|
| EAE | OX40/L−/− | Reduced CNS disease, CD4 T cells, IFN-γ, IL-2, IL-6 |
| OX40L block | Reduced CNS disease, CD4 T cells | |
| OX40L-Tg | Enhanced CNS disease | |
| Lung inflammation | OX40/L−/− | Reduced asthma, eosinophils, CD4 T cells, Th2 cytokines |
| OX40L block | Reduced asthma, eosinophils, CD4 T cells, Th2 cytokines | |
| OX40L-Tg | Spontaneous interstitial pneumonia | |
| Diabetes (NOD) | OX40L−/− | No pancreatic islet destruction, no increased blood glucose |
| OX40L block | Reduced diabetes incidence, insulitis | |
| Atherosclerosis | OX40L−/− | Reduced lesions |
| OX40L block | Reduced lesions, Th2 cytokines | |
| OX40L-Tg | Enhanced lesions | |
| GVHD/transplantation | OX40/L−/− | Reduced disease, rejection, infiltration, CD4/CD8 T cells |
| OX40L block | Reduced disease, infiltration, CD4/CD8 T cells, B cells, IFN-γ | |
| IBD/colitis | OX40L block | Decreased gut infiltration, CD4/CD8 T cells, cDC, IFN-γ |
| OX40L-Tg | Spontaneous gut infiltration | |
| Contact hypersensitivity | OX40L−/− | Reduced inflammation, CD4 T cells |
| OX40L-Tg | Enhanced inflammation, T cell response | |
| Arthritis | OX40L block | Reduced joint swelling, CD4 T cells, IFN-γ |
| EAC | OX40L block | Reduced eosinophilia, IL-5 |
| HgCl2 autoimmunity | OX40L block | Reduced Th2 cytokines, weight loss, mortality |
| Viral—LCMV | OX40−/− | Normal primary and memory CD8, normal Ig |
| VSV | OX40−/− | Normal Ig |
| Theilers V | OX40−/− | Normal primary CD8, normal Ig |
| Influenza V | OX40/L−/− | Normal primary CD8, reduced CD4 and memory CD8 |
| OX40L block | Reduced lung inflammation, CD4 and CD8 T cells | |
| mCMV | OX40−/− | Normal primary CD8, reduced memory CD8 and CD4 |
| VACV | OX40−/− | Reduced primary CD8/CD4, reduced memory CD8 and CD4 |
| Parasite | ||
| Heligmosomoides polygyrus | OX40L−/− | Decreased worm expulsion, reduced Th2 cytokines |
| Nippostrongylus brasiliensis | OX40−/− | Normal Th2 cytokines |
| Leishmania major | OX40L block | Reduced disease, decreased Th2 cytokines |
| OX40L-Tg | Enhanced susceptibility to disease, increased Th2 cytokines | |
| Listeria monocytogenes | OX40−/− | Normal primary CD8, reduced memory CD8 |
Abbreviations: EAC, experimental allergic conjunctivitis; EAE, experimental allergic encephalomyelitis; GVHD, graft-versus-host disease; IBD, inflammatory bowel disease; LCMV, lymphocytic choriomeningitis virus; mCMV, mouse cytomegalovirus; OX40/L−/−, OX40- and OX40L-deficient; OX40L-Tg, OX40L transgenic; VACV, vaccinia virus; VSV, vesicular stomatitis virus.
REGULATION OF CD8 T CELLS
Studies of CD8 T cells have generally lagged behind those of CD4 T cells. As noted above, most of the initial pioneering work on OX40 and OX40L was directed to CD4 T cells, and the reports that addressed the activity of CD8 T cells largely yielded negative data. For example, OX40L transgenic animals did not demonstrate any appreciably enhanced accumulation of activated CD8 T cells (51, 52), and brief analyses of OX40 and OX40L knockouts infected with three viruses (LCMV, influenza, and Theiler’s murine encephalomyelitis virus) did not reveal a pronounced defect in the generation of cytotoxic T lymphocytes (CTLs) (42, 44). This finding led some to suggest that OX40-OX40L interactions did not regulate CD8 T cells. Only with more defined systems and in-depth studies have investigators come to the conclusion that these molecules can also strongly dictate the magnitude of CD8 T cell responses.
The initial data showing that OX40-OX40L interactions directly enhanced priming of CD8 T cells came from systems where OX40-deficient TCR transgenic CD8 T cells were tracked in vivo responding to antigen in adjuvant, expressed on tumor cells, and delivered by nonreplicating adenovirus (12, 88, 89). Each showed defective expansion or survival of the CD8 T cells, largely mimicking data in CD4 systems. Thus, OX40 likely plays an analogous role on CD8 T cells compared with that on CD4 T cells, although no signaling studies have yet been performed to prove definitively that there are common intracellular targets. Studies of human CD8 T cells in vitro have supported this conclusion, showing that OX40 can be induced within 1–2 days of activation and that OX40L expressed on DCs can support growth and differentiation of these cells (90). Other data have shown defects in CD8 T cell responses in OX40L-deficient mice in contact hypersensitivity reactions and allograft rejection (15, 43), although these results may reflect an action on CD4 T cells that may be required to help the CTL response. Furthermore, there are now many reports of increased accumulation or activity of CD8 T cells in mice injected with agonist antibodies to OX40, either in simple systems or with viral infection or tumor inoculation. Although unclear, some of these findings might reflect a direct action of the antibody on the CD8 T cell (81, 88, 91–93); however, several studies also showed that depleting CD4 T cells abrogated CTL priming (94–98). This again suggests that OX40 can function on two levels to augment CD8 immunity directly and indirectly through CD4 T cells. How OX40 enhances CD4 T cell help for CD8 T cells has not been studied.
Whereas the initial reports of pathogens provided negative data with regard to OX40-OX40L, more recent studies have focused on long-term CD8 memory and recall immunity and found strong roles. One report of influenza virus infection demonstrated reduced accumulation of memory CD8 T cells in OX40L-deficient animals, along with reduced secondary expansion of these cells, even though primary CTL generation was normal (99). Blocking OX40-OX40L interactions also prevented weight loss and cachexia induced by intranasal influenza infection, which correlated with reduced accumulation of CD4 and CD8 T cells within the lung (100). Similarly, antigen-specific CD8 T cell populations that persisted long term after mouse cytomegalovirus (mCMV) or Listeria monocytogenes infection were reduced in size in OX40-deficient mice, even though acute virus- and bacteria-reactive CD8 populations developed normally (97, 101). A variation of this was found with vaccinia virus (VACV), in which case defective memory generation was again apparent, but the primary CD8 T cell response was also compromised in the absence of OX40 (13). Furthermore, in human in vitro systems, OX40 signals can directly enhance the expansion of several virus-specific memory CD8 T cell populations (HIV, EBV, flu), although its effects are mild without synergistic signals from the co-stimulatory molecules CD28 and 4-1BB (102), or again OX40 can indirectly promote CD8 T cell priming through augmenting CD4 T cell help (35). Even though a common theme is emerging that OX40-OX40L interactions often are necessary for CD8 memory, persistence of CD8 T cells, and recall activity, it is not clear why they are only sometimes required for the initial development of antigen-reactive CD8 T cell populations (Table 2). The use of OX40 likely depends on the length of time it is expressed on CD8 T cells. This in turn likely depends on the availability of CD4 T cell help to augment CTL generation and on the availability or extent of signaling of inflammatory cytokines such as IL-7, IL-15, and IFN-α. The latter control responses to infectious agents and are required for T cell longevity, and signals from their receptors might directly or indirectly affect OX40 expression or OX40 signaling on the CD8 T cell.
CONTROL OF OTHER INFLAMMATORY CELL TYPES
OX40 or OX40L can be expressed on a number of other cell types that control aspects of immune functionality (Figure 2). As noted above, OX40L has been found in vivo or in vitro on cells as diverse as mast cells, smooth muscle cells, vascular endothelium, and LTi cells. The most obvious role that this OX40L might serve would be to provide signals through OX40 to conventional CD4 and CD8 T cells at inflammatory sites to maintain or promote the T cell response. This could primarily involve the antigen-independent NF-κB signal that OX40-OX40L interactions can generate, although antigen presentation to CD8 T cells is a possibility, and reports of inducible class II MHC expression by some of these cell types imply presentation is also possible to CD4 T cells. Certainly, in vitro studies have shown that mast cells (25, 26), LTi cells (31, 103), and endothelial cells (57, 104) can provide costimulatory signals from OX40L for enhancing proliferation, survival, or cytokine production by T cells, although in vivo studies demonstrating these phenomena are lacking. OX40L reverse signaling into these cells might also control aspects of their cellular metabolism, although data related to this are limited to, for example, artificial crosslinking studies showing induction of chemokines such as CCL5 by endothelial cells (87, 105).
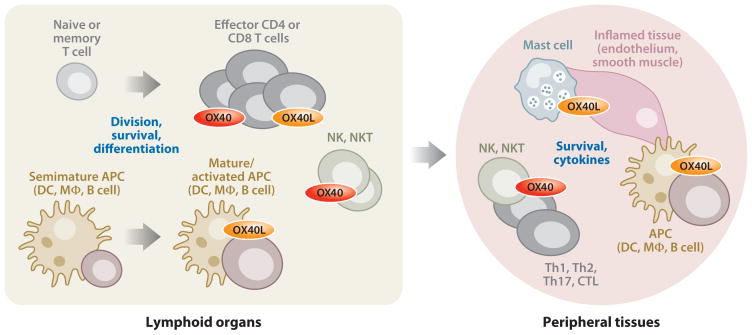
Figure 2.
OX40-OX40L interactions regulate functional activity of effector CD4 and CD8 T cells, NK cells, and NKT cells. OX40 is not expressed on naive or memory CD4 or CD8 T cells but is induced after antigen recognition. Similarly, OX40L is inducible on professional APCs (DCs, macrophages, B cells) after they mature and receive activating stimuli. The interaction of these cell types primarily allows OX40 to engage its signaling pathways, targeting division and survival proteins in the activated T cells, which leads to clonal expansion and the development of high frequencies of effector cells. OX40 may also regulate differentiation in the T cells depending on other inflammatory factors. Reverse signaling through OX40L to the APC most likely also contributes to these processes through inducing inflammatory cytokines. Interactions between NK cells and T cells or APCs, or between NKT cells and APCs, can also involve OX40-OX40L interactions, promoting direct cellular activity to NK and NKT cells and perhaps survival signals, as well as provide cytokine feedback loops that will further augment T cell priming. Within inflamed tissue, varied cell types have the potential to express OX40L, including activated endothelium, smooth muscle, and innate cells such as mast cells. Through additional bi-directional interactions with OX40, this OX40L likely potentiates local tissue inflammation by maintaining the effector cells and leading to production of proinflammatory events in the tissue-resident cells.
Activated NK cells also express OX40L (24, 106), which again can provide costimulatory signals to CD4 T cells in vitro (24). OX40-OX40L interactions can also augment NK function. OX40 was visualized on NK cells, and coculture of these cells with activated pDCs that expressed OX40L resulted in IFN-γ production, implying a direct effect of OX40 signals into the NK cell, but also potentially related to reverse signaling through OX40L on pDCs (20).
In line with this finding, several recent studies have focused on control of invariant NKT cells. Similar to the case with NK cells, coculture of pDCs with NKT cells resulted in OX40-dependent IFN-γ production (107), and cDCs that expressed OX40L and presented lipid antigen in the context of CD1d promoted OX40-dependent IFN-γ from NKT cells in vivo in a tumor model (108). As an extension of this crossregulation, OX40-OX40L interactions were also required for local production of IFN-α by pDC in a model of LCMV infection, with the implication that OX40 is provided by NKT cells in this setting and that the active signal is through OX40L to pDC (86). Lastly, other data have highlighted the potential for OX40 to control neutrophil activity. Ligation of OX40 on human neutrophils resulted in enhanced survival, which correlated with reduced activation of caspase 3, as well as with augmented levels of antiapoptotic and suppressed cleavage of proapoptotic Bcl-2 members (109). Although these data on NK cells, NKT cells, and neutrophils are not extensive, they certainly warrant additional attention and imply a broad base for how OX40-OX40L interactions might control both adaptive and innate immunity.
OX40 AS A THERAPEUTIC ADJUVANT
The strong activity of OX40-OX40L interactions in driving CD4 and CD8 T cells and the insights into augmentation of NK and NKT cell activity suggest that OX40 is a potential adjuvant that could be used as a target in vaccination strategies or therapeutic applications to promote protection against pathogens. Although control of acute viral replication, as alluded to above, is not affected by the inhibition of endogenous OX40-OX40L, and although control of initial parasite burden in several models with helminths is variably dependent on these molecules (42, 110–112), reagents that promote signals through OX40 are nonetheless attractive for enhancing protective immunity to infection. Several examples suggest promise in this area. With pulmonary growth of Cryptococcus neoformans, treatment with a stimulatory OX40L.Ig fusion protein promoted fungal clearance (113). Immunotherapy with OX40L.Ig in combination with anti-CTLA4 enhanced CD4 T cell responses, granuloma formation, and killing of Leishmania donovani (114). Targeting OX40 alone or with another TNFR family member, 4-1BB, during vaccination with a poxvirus vector expressing a nominal antigen strongly enhanced memory T cell responses to that antigen (96), a finding that is in line with earlier work showing that agonist antibodies to OX40 enhanced memory generation (11, 49). Anti-OX40 also promoted SIV gp130-specific T cell and antibody responses in rhesus monkeys (60), and, similarly, inclusion of the OX40L gene in a plasmid DNA vaccine encoding hepatitis B surface antigen (HBsAg) enhanced primary CD4 and CD8 T cell responses against this antigen, as well as long-term memory (115). Additionally, agonist antibodies to OX40 elicit strong antiviral CD4 and CD8 T cell responses when injected at the time of infection with live mCMV, resulting in enhanced clearance of the virus (97, 116). Lastly, recent data found that protection against lethal challenge with live VACV, after vaccination with a CD8 T cell epitope of VACV, was fully dependent on endogenous OX40-OX40L interactions (13), also suggesting that targeting OX40 would further promote protective memory if agonist reagents were given as part of a vaccine.
Another obvious application of OX40 agonist strategies is to augment antiself responses to tumor-associated antigens. This is an area that has received strong interest over the past eight or nine years, initiated by studies from Weinberg that showed OX40 was expressed on tumor-infiltrating T cells from patients with head and neck carcinoma or melanoma (117), and subsequently by studies showing that agonists of OX40 could augment protection against growth of melanoma, breast and colon carcinoma, and sarcoma in the mouse (118). These findings have been expanded in many tumor models to thymoma, glioma, B lymphoma, renal carcinoma, and prostate cancer. The tumor literature has been reviewed elsewhere (33, 119) and is not reiterated here except to point out that the effectiveness of targeting OX40 in isolation has been quite variable depending on the model system (solid tumor, metastases, prophylactic versus therapeutic treatment, highly immunogenic versus weakly immunogenic). Agonist antibodies to OX40 are currently in phase I clinical trials for cancer, but recent and current efforts are being focused on combining OX40 agonists, or tumor transfection protocols to express OX40L, with other forms of treatment, such as DC vaccines, adoptive T cell immunotherapy, and treatment with cytokines such as GM-CSF and IL-12. Because of the complexity of eliciting tumor-reactive T cells, along with NK and NKT cells that might be required for effective targeting of a tumor, in a cancer setting OX40 will likely only be a truly effective adjuvant if combined with other immune-based treatments. New soluble reagents are being developed, such as RNA aptamers that bind OX40 (120) and oligomeric OX40L.Ig fusion proteins (121, 122), which might be promising if they exhibit enhanced agonist activity.
As discussed briefly above, one advantage of targeting OX40 in cancer, or as an adjuvant for infectious disease, is the idea that its signals can antagonize the induction of adaptive Tregs (74, 75, 77). Moreover, other literature has indicated that OX40 signaling can prevent suppression mediated by already differentiated adaptive or thymus-derived CD25+Foxp3+ Tregs, either simply by making effector T cells resistant to suppression or by directly blocking Treg suppressive activity. The latter has been seen in basic model systems (75, 123, 124) as well as in a recent tumor model (125). However, one potential complication worth mentioning in terms of adjuvant therapy also relates to Tregs and to several studies that have shown some activity of OX40 in promoting the growth or survival of these cells (124, 126, 127), analogous to its effects on conventional CD4 and CD8 T cells. Adult OX40 and OX40L knockout animals essentially have normal numbers of Foxp3+ Tregs, suggesting that OX40 is not a natural survival factor for this subset. However, reagents that target OX40 may have concomitant effects in both transiently blocking suppressive activity as well as promoting the expansion or survival of these cells. Depending on the predominant and relative activity of OX40 signals to conventional T cells, NKT cells, and NK cells, a stimulatory action on Tregs might then impair the ability of agonist reagents to function effectively as adjuvants. However, given the well-known strong activity of OX40 agonists in promoting expansion of conventional CD4 and CD8 T cells and immunity in both basic and applied systems, a positive effect on Tregs may be a minor issue. This was illustrated in a recent paper in a tumor model in which anti-OX40 combined with cyclophosphamide-induced expansion of Foxp3+ Treg numbers when analyzed in peripheral tissues. However, fewer Tregs were found to accumulate at the tumor site in the same mice, and there was also a substantial increase in CD8 effector T cells, which correlated with a strong therapeutic activity of this combination treatment on tumor growth (128).
OX40 AND OX40L AS THERAPEUTIC TARGETS FOR INFLAMMATORY DISEASE
Lastly, research over the past 10 years has definitively shown the importance of OX40-OX40L interactions in development of immune-mediated disease. Most mouse models of autoimmunity and inflammatory disease have now been analyzed using either knockout mice or reagents that block OX40L. These studies have yielded results analogous to, and as impressive as, those that focused on CD28-B7 and CD40-CD40L interactions and that resulted in testing of CTLA4.Ig and anti-CD40L in a number of clinical trials. A strong reduction in disease severity or a complete lack of disease has been reported when OX40 or OX40L is absent or neutralized in EAE (MBP and PLP models), allergic asthma (OVA- and TSLP-induced models), colitis (RAG transfer, IL-2 knockout, hapten, and DSS models), diabetes (NOD and BDC2.5 transgenic models), arthritis (collagen-induced, IL-1Ra knockout, and adjuvant models), atherosclerosis (high fat diet–induced and LDLR knockout models), GVHD (acute and chronic models), and allograft rejection (minor and major MHC mismatches). Again, I have omitted the details and references for these studies owing to space limitations, but they can be found in other reviews (33, 119), with a summary of the data in Table 2. Polymorphisms in OX40L have also now been linked to susceptibility to atherosclerosis (129) and SLE (130), although to date no study of OX40 or OX40L has been performed in mouse models of the latter. Encouragingly, a number of reports have shown that therapeutic targeting of OX40L can block ongoing disease in EAE (21), colitis (45, 131, 132), arthritis (48), asthma (55, 61), and diabetes (133) and that depletion of OX40 positive cells by drug/toxin delivery can also prevent disease in EAE (39) and arthritis (134) models. Here, the effect of OX40-OX40L interactions on Treg induction, maintenance, and function should again be highlighted. Assuming that a major physiological activity of OX40 signals is to suppress the generation of adaptive Tregs and to block suppression from both adaptive and thymus-derived Tregs, it follows that this will be a great advantage in terms of therapeutic inhibition of the OX40-OX40L interaction. Pathogenic effector cells (T cells, NK cells, NKT cells) will be neutralized, while allowing Tregs still to exist or be newly generated and retain functionality. The control of Tregs by OX40 has not yet been extensively investigated in autoimmune or inflammatory disease situations, but recent data in asthma and transplant models support this hypothesis (76, 78).
Neutralizing antibodies to OX40L are currently in phase I clinical trials for asthma, and, depending on success, most likely will be tested in other inflammatory diseases. One potential issue concerns a recent claim that OX40L was expressed on platelets from patients with acute coronary syndrome (135). Platelet expression of OX40L needs to be confirmed, but, given the prothrombotic complications that arose after treatment of patients with antibodies to CD40L, another TNF superfamily molecule that was also found on platelets, this should be followed up in future analyses.
SUMMARY POINTS
OX40 and OX40L are not ubiquitously expressed. They are induced on a number of lymphoid and nonlymphoid cell types, correlating with their activities in enhancing ongoing inflammation and immune function.
OX40-OX40L interactions exert several effects on conventional CD4 and CD8 T cells, NK cells, and NKT cells, including promoting division, survival, and differentiation, and regulating cytokine production. OX40-OX40L interactions additionally modulate the differentiation and activity of regulatory T cells.
OX40 targets intracellular signaling mediators that control canonical and noncanonical NF-κB, PI3K/Akt, as well as calcium/NFAT pathways. OX40L can also promote signals in APCs and nonlymphoid cells that are not yet defined but likely control production of proinflammatory cytokines
Many studies have analyzed the effects of removing or neutralizing OX40-OX40L interactions in autoimmune disease, inflammation, and infectious disease models with strong results. Blocking these molecules holds great promise for therapeutic manipulation of immune disease.
Agonist reagents to OX40 are strong adjuvants that allow development of protective T cells that can target tumors as well as pathogens.
Acknowledgments
I would like to acknowledge all the laboratories that have contributed to the literature on OX40 and OX40L. Many references could not be included because of space restrictions, but this does not diminish their importance or value. M.C. is supported by grants AI67341, CA91837, AI49453, and AI070535 from the National Institutes of Health. This is publication #1145 from the La Jolla Institute for Allergy and Immunology.
- cDC/pDC
conventional/plasmacytoid dendritic cells
- EAE
experimental allergic encephalomyelitis
- MS
multiple sclerosis
- RA
rheumatoid arthritis
- GVHD
graft-versus-host disease
- TRAF
TNF-associated factor
- LCMV
lymphocytic choriomeningitis virus
- mCMV
mouse cytomegalovirus
- VACV
vaccinia virus
Footnotes
DISCLOSURE STATEMENT
M.C. has patents on OX40 and OX40L.
LITERATURE CITED
- 1.Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, et al. Antigens of activated rat T lymphocytes including a molecule of 50000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24:1281–90. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 2.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes—a molecule related to nerve growth factor receptor. EMBO J. 1990;9:1063–68. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calderhead DM, Buhlmann JE, van den Eertwegh, Claassen E, Noelle RJ, Fell HP. Cloning of mouse Ox40: a T cell activation marker that may mediate T-B cell interactions. J Immunol. 1993;151:5261–71. [PubMed] [Google Scholar]
- 4.Latza U, Durkop H, Schnittger S, Ringeling J, Eitelbach F, et al. The human OX40 homolog: cDNA structure, expression and chromosomal assignment of the ACT35 antigen. Eur J Immunol. 1994;24:677–83. doi: 10.1002/eji.1830240329. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka Y, Inoi T, Tozawa H, Yamamoto N, Hinuma Y. A glycoprotein antigen detected with new monoclonal antibodies on the surface of human lymphocytes infected with human T-cell leukemia virus type-I (HTLV-I) Int J Cancer. 1985;36:549–55. doi: 10.1002/ijc.2910360506. [DOI] [PubMed] [Google Scholar]
- 6.Miura S, Ohtani K, Numata N, Niki M, Ohbo K, et al. Molecular cloning and characterization of a novel glycoprotein, gp34, that is specifically induced by the human T-cell leukemia virus type I transactivator p40tax. Mol Cell Biol. 1991;11:1313–25. doi: 10.1128/mcb.11.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum PR, Gayle RB, Ramsdell F, Srinivasan S, Sorensen RA, et al. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 1994;13:3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimojima M, Miyazawa T, Ikeda Y, McMonagle EL, Haining H, et al. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science. 2004;303:1192–95. doi: 10.1126/science.1092124. [DOI] [PubMed] [Google Scholar]
- 9.Compaan DM, Hymowitz SG. The crystal structure of the costimulatory OX40-OX40L complex. Structure. 2006;14:1321–30. doi: 10.1016/j.str.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Gramaglia I, Weinberg AD, Lemon M, Croft M. OX40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–17. [PubMed] [Google Scholar]
- 11.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–50. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 12.Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172:4821–25. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 13.Salek-Ardakani S, Moutaftsi M, Crotty S, Sette A, Croft M. OX40 drives protective vaccinia virus-specific CD8 T cells. J Immunol. 2008;181:7969–76. doi: 10.4049/jimmunol.181.11.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuber E, Neurath M, Calderhead D, Fell HP, Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–21. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 15.Murata K, Ishii N, Takano H, Miura S, Ndhlovu LC, et al. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J Exp Med. 2000;191:365–74. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–48. [PubMed] [Google Scholar]
- 17.Tanaka H, Demeure CE, Rubio M, Delespesse G, Sarfati M. Human monocyte-derived dendritic cells induce naive T cell differentiation into T helper cell type 2 (Th2) or Th1/Th2 effectors. Role of stimulator/responder ratio. J Exp Med. 2000;192:405–12. doi: 10.1084/jem.192.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato T, Ishii N, Murata K, Kikuchi K, Nakagawa S, et al. Consequences of OX40-OX40 ligand interactions in Langerhans cell function: enhanced contact hypersensitivity responses in OX40L-transgenic mice. Eur J Immunol. 2002;32:3326–35. doi: 10.1002/1521-4141(200211)32:11<3326::AID-IMMU3326>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Amakawa R, Inaba M, Hori T, Ota M, et al. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J Immunol. 2004;172:4253–59. doi: 10.4049/jimmunol.172.7.4253. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Lou Y, Lizee G, Qin H, Liu S, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Investig. 2008;118:1165–75. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol. 1999;162:1818–26. [PubMed] [Google Scholar]
- 22.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maxwell JR, Yadav R, Rossi RJ, Ruby CE, Weinberg AD, et al. IL-18 bridges innate and adaptive immunity through IFN-γ and the CD134 pathway. J Immunol. 2006;177:234–45. doi: 10.4049/jimmunol.177.1.234. [DOI] [PubMed] [Google Scholar]
- 24.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173:3716–24. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 25.Kashiwakura J, Yokoi H, Saito H, Okayama Y. T cell proliferation by direct cross-talk between OX40 ligand on human mast cells and OX40 on human T cells: comparison of gene expression profiles between human tonsillar and lung-cultured mast cells. J Immunol. 2004;173:5247–57. doi: 10.4049/jimmunol.173.8.5247. [DOI] [PubMed] [Google Scholar]
- 26.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–48. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 27.Imura A, Hori T, Imada K, Ishikawa T, Tanaka Y, et al. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183:2185–95. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess JK, Carlin S, Pack RA, Arndt GM, Au WW, et al. Detection and characterization of OX40 ligand expression in human airway smooth muscle cells: a possible role in asthma? J Allergy Clin Immunol. 2004;113:683–89. doi: 10.1016/j.jaci.2003.12.311. [DOI] [PubMed] [Google Scholar]
- 29.Takasawa N, Ishii N, Higashimura N, Murata K, Tanaka Y, et al. Expression of gp34 (OX40 ligand) and OX40 on human T cell clones. Jpn J Cancer Res. 2001;92:377–82. doi: 10.1111/j.1349-7006.2001.tb01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soroosh P, Ine S, Sugamura K, Ishii N. OX40-OX40 ligand interaction through T cell-T cell contact contributes to CD4 T cell longevity. J Immunol. 2006;176:5975–87. doi: 10.4049/jimmunol.176.10.5975. [DOI] [PubMed] [Google Scholar]
- 31.Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, et al. CD4+CD3− accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–54. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 32.Kim MY, Anderson G, White A, Jenkinson E, Arlt W, et al. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3− inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J Immunol. 2005;174:6686–91. doi: 10.4049/jimmunol.174.11.6686. [DOI] [PubMed] [Google Scholar]
- 33.Salek-Ardakani S, Song A, Humphreys IR, Croft M. OX40:OX40L axis: emerging targets for immunotherapy of human disease. Curr Immunol Rev. 2006;2:37–53. [Google Scholar]
- 34.Giacomelli R, Passacantando A, Perricone R, Parzanese I, Rascente M, et al. T lymphocytes in the synovial fluid of patients with active rheumatoid arthritis display CD134-OX40 surface antigen. Clin Exp Rheumatol. 2001;19:317–20. [PubMed] [Google Scholar]
- 35.Yu Q, Yue FY, Gu XX, Schwartz H, Kovacs CM, Ostrowski MA. OX40 ligation of CD4+ T cells enhances virus-specific CD8+ T cell memory responses independently of IL-2 and CD4+ T regulatory cell inhibition. J Immunol. 2006;176:2486–95. doi: 10.4049/jimmunol.176.4.2486. [DOI] [PubMed] [Google Scholar]
- 36.Endl J, Rosinger S, Schwarz B, Friedrich SO, Rothe G, et al. Coexpression of CD25 and OX40 (CD134) receptors delineates autoreactive T-cells in type 1 diabetes. Diabetes. 2006;55:50–60. [PubMed] [Google Scholar]
- 37.Godfrey WR, Fagnoni FF, Harara MA, Buck D, Engleman EG. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J Exp Med. 1994;180:757–62. doi: 10.1084/jem.180.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg AD, Wallin JJ, Jones RE, Sullivan TJ, Bourdette DN, et al. Target organ-specific up-regulation of the MRC OX-40 marker and selective production of Th1 lymphokine mRNA by encephalitogenic T helper cells isolated from the spinal cord of rats with experimental autoimmune encephalomyelitis. J Immunol. 1994;152:4712–21. [PubMed] [Google Scholar]
- 39.Weinberg AD, Bourdette DN, Sullivan TJ, Lemon M, Wallin JJ, et al. Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nat Med. 1996;2:183–89. doi: 10.1038/nm0296-183. [DOI] [PubMed] [Google Scholar]
- 40.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akiba H, Oshima H, Takeda K, Atsuta M, Nakano H, et al. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J Immunol. 1999;162:7058–66. [PubMed] [Google Scholar]
- 42.Pippig SD, Pena-Rossi C, Long J, Godfrey WR, Fowell DJ, et al. Robust B cell immunity but impaired T cell proliferation in the absence of CD134 (OX40) J Immunol. 1999;163:6520–29. [PubMed] [Google Scholar]
- 43.Chen AI, McAdam AJ, Buhlmann JE, Scott S, Lupher ML, Jr, et al. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689–98. doi: 10.1016/s1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- 44.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, et al. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 45.Higgins LM, McDonald SA, Whittle N, Crockett N, Shields JG, MacDonald TT. Regulation of T cell activation in vitro and in vivo by targeting the OX40-OX40 ligand interaction: amelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J Immunol. 1999;162:486–93. [PubMed] [Google Scholar]
- 46.Akiba H, Miyahira Y, Atsuta M, Takeda K, Nohara C, et al. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J Exp Med. 2000;191:375–80. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsukada N, Akiba H, Kobata T, Aizawa Y, Yagita H, Okumura K. Blockade of CD134 (OX40)-CD134L interaction ameliorates lethal acute graft-versus-host disease in a murine model of allogeneic bone marrow transplantation. Blood. 2000;95:2434–39. [PubMed] [Google Scholar]
- 48.Yoshioka T, Nakajima A, Akiba H, Ishiwata T, Asano G, et al. Contribution of OX40/OX40 ligand interaction to the pathogenesis of rheumatoid arthritis. Eur J Immunol. 2000;30:2815–23. doi: 10.1002/1521-4141(200010)30:10<2815::AID-IMMU2815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 49.Maxwell J, Weinberg AD, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–12. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 50.Bansal-Pakala P, Gebre-Hiwot Jember A, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7:907–12. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 51.Brocker T, Gulbranson-Judge A, Flynn S, Riedinger M, Raykundalia C, Lane P. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur J Immunol. 1999;29:1610–16. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 52.Murata K, Nose M, Ndhlovu LC, Sato T, Sugamura K, Ishii N. Constitutive OX40/OX40 ligand interaction induces autoimmune-like diseases. J Immunol. 2002;169:4628–36. doi: 10.4049/jimmunol.169.8.4628. [DOI] [PubMed] [Google Scholar]
- 53.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–55. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 54.Song J, Salek-Ardakani S, Rogers PR, Cheng M, Van Parijs L, Croft M. The costimulation-regulated duration of PKB activation controls T cell longevity. Nat Immunol. 2004;5:150–58. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- 55.Salek-Ardakani S, Song J, Halteman BS, Jember AG, Akiba H, et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198:315–24. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soroosh P, Ine S, Sugamura K, Ishii N. Differential requirements for OX40 signals on generation of effector and central memory CD4+ T cells. J Immunol. 2007;179:5014–23. doi: 10.4049/jimmunol.179.8.5014. [DOI] [PubMed] [Google Scholar]
- 57.Kunitomi A, Hori T, Imura A, Uchiyama T. Vascular endothelial cells provide T cells with costimulatory signals via the OX40/gp34 system. J Leukoc Biol. 2000;68:111–18. [PubMed] [Google Scholar]
- 58.Ukyo N, Hori T, Yanagita S, Ishikawa T, Uchiyama T. Costimulation through OX40 is crucial for induction of an alloreactive human T-cell response. Immunology. 2003;109:226–31. doi: 10.1046/j.1365-2567.2003.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kober J, Leitner J, Klauser C, Woitek R, Majdic O, et al. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur J Immunol. 2008;38:2678–88. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weinberg AD, Thalhofer C, Morris N, Walker JM, Seiss D, et al. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J Immunother. 2006;29:575–85. doi: 10.1097/01.cji.0000211319.00031.fc. [DOI] [PubMed] [Google Scholar]
- 61.Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Investig. 2007;117:3868–78. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawamata S, Hori T, Imura A, Takaori-Kondo A, Uchiyama T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-κB activation. J Biol Chem. 1998;273:5808–14. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- 63.Arch RH, Thompson CB. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor κB. Mol Cell Biol. 1998;18:558–65. doi: 10.1128/mcb.18.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye H, Park YC, Kreishman M, Kieff E, Wu H. The structural basis for the recognition of diverse receptor sequences by TRAF2. Mol Cell. 1999;4:321–30. doi: 10.1016/s1097-2765(00)80334-2. [DOI] [PubMed] [Google Scholar]
- 65.Song J, So T, Croft M. Activation of NF-κB1 by OX40 contributes to antigen-driven T cell expansion and survival. J Immunol. 2008;180:7240–48. doi: 10.4049/jimmunol.180.11.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–31. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Song J, Salek-Ardakani S, So T, Croft M. The kinases aurora B and mTOR regulate the G1-S cell cycle progression of T lymphocytes. Nat Immunol. 2007;8:64–73. doi: 10.1038/ni1413. [DOI] [PubMed] [Google Scholar]
- 68.Linton PJ, Bautista B, Biederman E, Bradley ES, Harbertson J, et al. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–83. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.So T, Song J, Sugie K, Altman A, Croft M. Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc Natl Acad Sci USA. 2006;103:3740–45. doi: 10.1073/pnas.0600205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prell RA, Evans DE, Thalhofer C, Shi T, Funatake C, Weinberg AD. OX40-mediated memory T cell generation is TNF receptor-associated factor 2 dependent. J Immunol. 2003;171:5997–6005. doi: 10.4049/jimmunol.171.11.5997. [DOI] [PubMed] [Google Scholar]
- 71.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ, Weinberg AD, Parker DC. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–43. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 72.Williams CA, Murray SE, Weinberg AD, Parker DC. OX40-mediated differentiation to effector function requires IL-2 receptor signaling but not CD28, CD40, IL-12Rβ2, or T-bet. J Immunol. 2007;178:7694–702. doi: 10.4049/jimmunol.178.12.7694. [DOI] [PubMed] [Google Scholar]
- 73.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J Immunol. 2008;180:2140–48. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 74.So T, Croft M. Cutting edge: OX40 inhibits TGF-β- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–30. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 75.Vu MD, Xiao X, Gao W, Degauque N, Chen M, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–10. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen M, Xiao X, Demirci G, Li XC. OX40 controls islet allograft tolerance in CD154 deficient mice by regulating FOXP3+ Tregs. Transplantation. 2008;85:1659–62. doi: 10.1097/TP.0b013e3181726987. [DOI] [PubMed] [Google Scholar]
- 77.Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, et al. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci USA. 2006;103:13138–43. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duan W, So T, Croft M. Antagonism of airway tolerance by endotoxin/lipopolysaccharide through promoting OX40L and suppressing antigen-specific Foxp3+ T regulatory cells. J Immunol. 2008;181:8650–59. doi: 10.4049/jimmunol.181.12.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohshima Y, Yang LP, Uchiyama T, Tanaka Y, Baum P, et al. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4+ T cells into high IL-4-producing effectors. Blood. 1998;92:3338–45. [PubMed] [Google Scholar]
- 80.Rogers PR, Croft M. CD28, OX40, LFA-1, and CD4 modulation of Th1/Th2 differentiation is directly dependent on the dose of antigen. J Immunol. 2000;164:2955–63. doi: 10.4049/jimmunol.164.6.2955. [DOI] [PubMed] [Google Scholar]
- 81.De Smedt T, Smith J, Baum P, Fanslow W, Butz E, Maliszewski C. Ox40 costimulation enhances the development of T cell responses induced by dendritic cells in vivo. J Immunol. 2002;168:661–70. doi: 10.4049/jimmunol.168.2.661. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Li L, Shang X, Benson J, Merle Elloso M, et al. Negative regulation of IL-17 production by OX40/OX40L interaction. Cell Immunol. 2008;253:31–37. doi: 10.1016/j.cellimm.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 83.Ndhlovu LC, Ishii N, Murata K, Sato T, Sugamura K. Critical involvement of OX40 ligand signals in the T cell priming events during experimental autoimmune encephalomyelitis. J Immunol. 2001;167:2991–99. doi: 10.4049/jimmunol.167.5.2991. [DOI] [PubMed] [Google Scholar]
- 84.Horai R, Nakajima A, Habiro K, Kotani M, Nakae S, et al. TNF-α is crucial for the development of autoimmune arthritis in IL-1 receptor antagonist-deficient mice. J Clin Investig. 2004;114:1603–11. doi: 10.1172/JCI20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986–90. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diana J, Griseri T, Lagaye S, Beaudoin L, Autrusseau E, et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity. 2009;30:289–99. doi: 10.1016/j.immuni.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 87.Matsumura Y, Hori T, Kawamata S, Imura A, Uchiyama T. Intracellular signaling of gp34, the OX40 ligand: induction of c-jun and c-fos mRNA expression through gp34 upon binding of its receptor, OX40. J Immunol. 1999;163:3007–11. [PubMed] [Google Scholar]
- 88.Song A, Tang X, Harms KM, Croft M. OX40 and Bcl-xL promote the persistence of CD8 T cells to recall tumor-associated antigen. J Immunol. 2005;175:3534–41. doi: 10.4049/jimmunol.175.6.3534. [DOI] [PubMed] [Google Scholar]
- 89.Lee SW, Park Y, Song A, Cheroutre H, Kwon BS, Croft M. Functional dichotomy between OX40 and 4-1BB in modulating effector CD8 T cell responses. J Immunol. 2006;177:4464–72. doi: 10.4049/jimmunol.177.7.4464. [DOI] [PubMed] [Google Scholar]
- 90.Fujita T, Ukyo N, Hori T, Uchiyama T. Functional characterization of OX40 expressed on human CD8+ T cells. Immunol Lett. 2006;106:27–33. doi: 10.1016/j.imlet.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 91.Murata S, Ladle BH, Kim PS, Lutz ER, Wolpoe ME, et al. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol. 2006;176:974–83. doi: 10.4049/jimmunol.176.2.974. [DOI] [PubMed] [Google Scholar]
- 92.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the acquisition of CD8 T cell effector function after priming with tumor or soluble antigen can be overcome by the addition of an OX40 agonist. J Immunol. 2007;179:7244–53. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 93.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–15. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 94.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–21. [PubMed] [Google Scholar]
- 95.Lee SJ, Myers L, Muralimohan G, Dai J, Qiao Y, et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–12. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 96.Munks MW, Mourich DV, Mittler RS, Weinberg AD, Hill AB. 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunology. 2004;112:559–66. doi: 10.1111/j.1365-2567.2004.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Humphreys IR, Loewendorf A, de Trez C, Schneider K, Benedict CA, et al. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T cells: a CD4-dependent mechanism. J Immunol. 2007;179:2195–202. doi: 10.4049/jimmunol.179.4.2195. [DOI] [PubMed] [Google Scholar]
- 98.Song A, Song J, Tang X, Croft M. Cooperation between CD4 and CD8 T cells for antitumor activity is enhanced by OX40 signals. Eur J Immunol. 2007;37:1224–32. doi: 10.1002/eji.200636957. [DOI] [PubMed] [Google Scholar]
- 99.Hendriks J, Xiao Y, Rossen JW, Van Der Sluijs KF, Sugamura K, et al. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–76. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 100.Humphreys IR, Walzl G, Edwards L, Rae A, Hill S, Hussell T. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J Exp Med. 2003;198:1237–42. doi: 10.1084/jem.20030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mousavi SF, Soroosh P, Takahashi T, Yoshikai Y, Shen H, et al. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181:5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Serghides L, Bukczynski J, Wen T, Wang C, Routy JP, et al. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J Immunol. 2005;175:6368–77. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 103.Gaspal FM, Kim MY, McConnell FM, Raykundalia C, Bekiaris V, Lane PJ. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol. 2005;174:3891–96. doi: 10.4049/jimmunol.174.7.3891. [DOI] [PubMed] [Google Scholar]
- 104.Mestas J, Crampton SP, Hori T, Hughes CC. Endothelial cell costimulation through OX40 augments and prolongs T cell cytokine synthesis by stabilization of cytokine mRNA. Int Immunol. 2005;17:737–47. doi: 10.1093/intimm/dxh255. [DOI] [PubMed] [Google Scholar]
- 105.Kotani A, Hori T, Matsumura Y, Uchiyama T. Signaling of gp34 (OX40 ligand) induces vascular endothelial cells to produce a CC chemokine RANTES/CCL5. Immunol Lett. 2002;84:1–7. doi: 10.1016/s0165-2478(02)00082-2. [DOI] [PubMed] [Google Scholar]
- 106.Hanna J, Bechtel P, Zhai Y, Youssef F, McLachlan K, Mandelboim O. Novel insights on human NK cells’ immunological modalities revealed by gene expression profiling. J Immunol. 2004;173:6547–63. doi: 10.4049/jimmunol.173.11.6547. [DOI] [PubMed] [Google Scholar]
- 107.Marschner A, Rothenfusser S, Hornung V, Prell D, Krug A, et al. CpG ODN enhance antigen-specific NKT cell activation via plasmacytoid dendritic cells. Eur J Immunol. 2005;35:2347–57. doi: 10.1002/eji.200425721. [DOI] [PubMed] [Google Scholar]
- 108.Zaini J, Andarini S, Tahara M, Saijo Y, Ishii N, et al. OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumor immunity in mice. J Clin Investig. 2007;117:3330–38. doi: 10.1172/JCI32693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baumann R, Yousefi S, Simon D, Russmann S, Mueller C, Simon HU. Functional expression of CD134 by neutrophils. Eur J Immunol. 2004;34:2268–75. doi: 10.1002/eji.200424863. [DOI] [PubMed] [Google Scholar]
- 110.Ekkens MJ, Liu Z, Liu Q, Whitmire J, Xiao S, et al. The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J Immunol. 2003;170:384–93. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- 111.Ierna MX, Scales HE, Schwarz H, Bunce C, McIlgorm A, et al. OX40 interactions in gastrointestinal nematode infection. Immunology. 2006;117:108–16. doi: 10.1111/j.1365-2567.2005.02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Balic A, Harcus Y, Holland MJ, Maizels RM. Selective maturation of dendritic cells by Nippostrongylus brasiliensis-secreted proteins drives Th2 immune responses. Eur J Immunol. 2004;34:3047–59. doi: 10.1002/eji.200425167. [DOI] [PubMed] [Google Scholar]
- 113.Humphreys IR, Edwards L, Walzl G, Rae AJ, Dougan G, et al. OX40 ligation on activated T cells enhances the control of Cryptococcus neoformans and reduces pulmonary eosinophilia. J Immunol. 2003;170:6125–32. doi: 10.4049/jimmunol.170.12.6125. [DOI] [PubMed] [Google Scholar]
- 114.Zubairi S, Sanos SL, Hill S, Kaye PM. Immunotherapy with OX40L-Fc or anti-CTLA-4 enhances local tissue responses and killing of Leishmania donovani. Eur J Immunol. 2004;34:1433–40. doi: 10.1002/eji.200324021. [DOI] [PubMed] [Google Scholar]
- 115.Du X, Zheng G, Jin H, Kang Y, Wang J, et al. The adjuvant effects of costimulatory molecules on cellular and memory responses to HBsAg DNA vaccination. J Gene Med. 2007;9:136–46. doi: 10.1002/jgm.1004. [DOI] [PubMed] [Google Scholar]
- 116.Humphreys IR, de Trez C, Kinkade A, Benedict CA, Croft M, Ware CF. Cytomegalovirus exploits IL-10-mediated immune regulation in the salivary glands. J Exp Med. 2007;204:1217–25. doi: 10.1084/jem.20062424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vetto JT, Lum S, Morris A, Sicotte M, Davis J, et al. Presence of the T-cell activation marker OX-40 on tumor infiltrating lymphocytes and draining lymph node cells from patients with melanoma and head and neck cancers. Am J Surg. 1997;174:258–65. doi: 10.1016/s0002-9610(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 118.Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–69. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 119.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–85. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dollins CM, Nair S, Boczkowski D, Lee J, Layzer JM, et al. Assembling OX40 aptamers on a molecular scaffold to create a receptor-activating aptamer. Chem Biol. 2008;15:675–82. doi: 10.1016/j.chembiol.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morris NP, Peters C, Montler R, Hu HM, Curti BD, et al. Development and characterization of recombinant human Fc:OX40L fusion protein linked via a coiled-coil trimerization domain. Mol Immunol. 2007;44:3112–21. doi: 10.1016/j.molimm.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muller N, Wyzgol A, Munkel S, Pfizenmaier K, Wajant H. Activity of soluble OX40 ligand is enhanced by oligomerization and cell surface immobilization. FEBS J. 2008;275:2296–304. doi: 10.1111/j.1742-4658.2008.06382.x. [DOI] [PubMed] [Google Scholar]
- 123.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4+CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–51. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 124.Kroemer A, Xiao X, Vu MD, Gao W, Minamimura K, et al. OX40 controls functionally different T cell subsets and their resistance to depletion therapy. J Immunol. 2007;179:5584–91. doi: 10.4049/jimmunol.179.8.5584. [DOI] [PubMed] [Google Scholar]
- 125.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–39. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, et al. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–89. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 127.Hippen KL, Harker-Murray P, Porter SB, Merkel SC, Londer A, et al. Umbilical cord blood regulatory T-cell (Treg) expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112:2847–57. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–16. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang X, Ria M, Kelmenson PM, Eriksson P, Higgins DC, et al. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat Genet. 2005;37:365–72. doi: 10.1038/ng1524. [DOI] [PubMed] [Google Scholar]
- 130.Cunninghame Graham DS, Graham RR, Manku H, Wong AK, Whittaker JC, et al. Polymorphism at the TNF superfamily gene TNFSF4 confers susceptibility to systemic lupus erythematosus. Nat Genet. 2008;40:83–89. doi: 10.1038/ng.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Obermeier F, Schwarz H, Dunger N, Strauch UG, Grunwald N, et al. OX40/OX40L interaction induces the expression of CXCR5 and contributes to chronic colitis induced by dextran sulfate sodium in mice. Eur J Immunol. 2003;33:3265–74. doi: 10.1002/eji.200324124. [DOI] [PubMed] [Google Scholar]
- 132.Totsuka T, Kanai T, Uraushihara K, Iiyama R, Yamazaki M, et al. Therapeutic effect of anti-OX40L and anti-TNF-a MAbs in a murine model of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G595–603. doi: 10.1152/ajpgi.00450.2002. [DOI] [PubMed] [Google Scholar]
- 133.Pakala SV, Bansal-Pakala P, Halteman BS, Croft M. Prevention of diabetes in NOD mice at a late stage by targeting OX40/OX40 ligand interactions. Eur J Immunol. 2004;34:3039–46. doi: 10.1002/eji.200425141. [DOI] [PubMed] [Google Scholar]
- 134.Boot EP, Koning GA, Storm G, Wagenaar-Hilbers JP, van Eden W, et al. CD134 as target for specific drug delivery to auto-aggressive CD4+ T cells in adjuvant arthritis. Arthritis Res Ther. 2005;7:R604–15. doi: 10.1186/ar1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu DM, Yan JC, Wang CP, Chen GH, Ding S, et al. The clinical implications of increased OX40 ligand expression in patients with acute coronary syndrome. Clin Chim Acta. 2008;397:22–26. doi: 10.1016/j.cca.2008.07.003. [DOI] [PubMed] [Google Scholar]