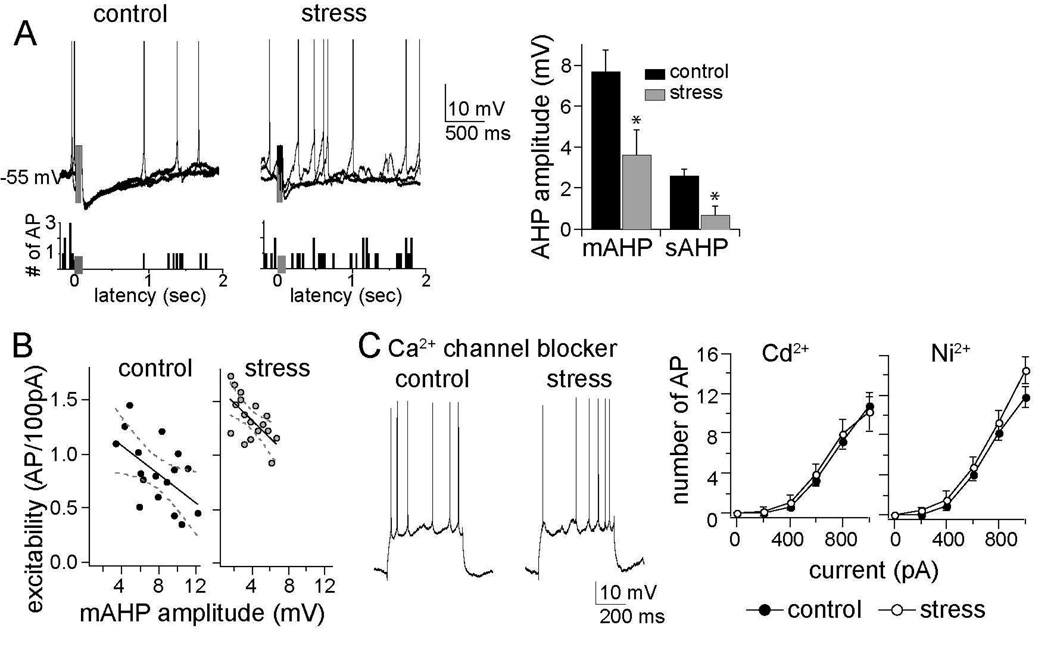
Figure 5. The AHP is reduced by chronic stress and necessary for the effects of chronic stress.
A) The amplitudes of both the medium- and slow AHP were reduced after chronic stress (sAHP control 2.6 ± 0.3 m V, n=17, stress 0.7 ± 0.4 m V, n=18, p=0.003, two-tailed unpaired t-test, t=3.26; mAHP control 7.7 ± 1.1 m V, n=21, stress 3.6 ± 1.2 m V, n=25, p=0.009, two-tailed unpaired t-test, t=2.72), as seen in the neuronal response to a burst of 5 action potentials evoked by 5 current pulses (presented at grey box in overlay of 3 consecutive voltage traces and firing rate histogram; action potentials are truncated during the burst for clarity, but see Figure S2 in Supplement 1 for details). This reduction of the AHP amplitude was associated with greater spontaneous firing near action potential threshold, demonstrated in a firing rate histogram of 10 consecutive sweeps. B) The amplitude of the mAHP was correlated with neuronal excitability (control, r=−0.56, r2=0.32; stress, r=−0.62, r2=0.39), demonstrating that it is a major factor in regulating LAT neuronal activity. C) Intracellular administration of Ca2+ channel blockers mimicked the effects of chronic stress on neuronal excitability: Cd2+ (mixed design repeated measures ANOVA of each stimulation intensity, main effect of stress F(1,66) = 0.153, p=0.697; main effect of stimulation intensity F(5,66) = 40.72, p<0.001; interaction F(5,66) = 0.124, p=0.987) and Ni2+ (mixed design repeated measures ANOVA of each stimulation intensity, main effect of stress F(1,72) = 3.77, p=0.056; main effect of stimulation intensity F(5,72) = 86.33, p<0.001; interaction F(5,72) = 0.711, p=0.617). Displayed 28 in this panel are traces with intracellular Cd 2+. * indicates p<0.05 between control and stress group in post-hoc unpaired t-tests with Bonferroni corrections.