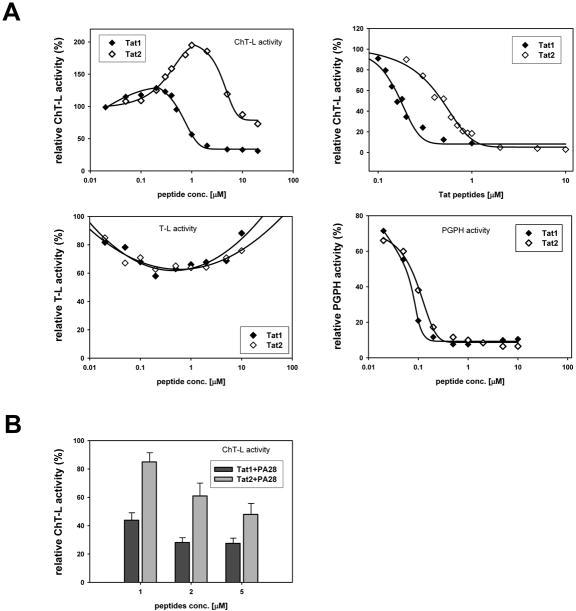
Figure 4.
Tat peptides affected selected peptidase activities of human 20S proteasome and its interactions with native PA28αβ activator. In all graphs 100% of the relative effect (y axis) refers to activity (nanomoles of released AMC product per mg of CP protein per second) recorded with a solvent (DMSO) without the Tat peptides added to reaction mixture.
(A) top left: Tat peptides exhibited a complex influence on ChT-L activity of CP. At lower concentrations Tat2 peptide, and to a much lesser extent Tat1, activated latent CP. Higher concentrations of the peptides inhibited the ChT-L peptidase. In contrast, this activity was inhibited by the whole range of peptides concentrations when CP was activated by 0.005% SDS (top right; I50=0.16 microM with Tat1 and 0.37 microM with Tat2). T-L activity of latent CP was only weakly affected by the peptides (bottom left). PGPH activity of latent CP was similarly inhibited by both the peptides (bottom right; I50=0.063 mM with Tat1 and 0.075 mM with Tat2). The data points are averages from 3 to 7 experiments, with standard deviations in the range of 7% to 15%.
(B) Tat peptides interfered with activation of ChT-L peptidase by native PA28αβ in a dose-dependent manner. Molar ratio of CP to PA28αβ was 1:3. (mean ± SD; n = at least 3)