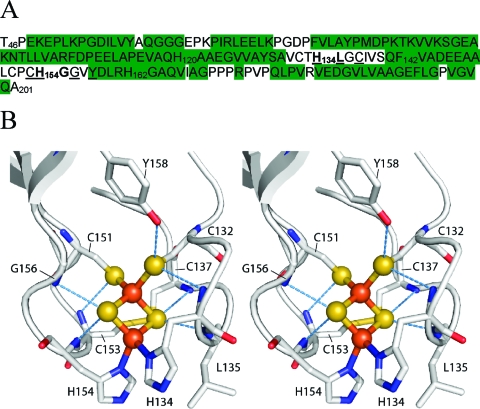
Figure 1.
Sequence and structure of TtRp. (A) Primary sequence of the water-soluble fragment of the T. thermophilus Rieske protein (TtRp) investigated here. The construct was truncated to remove the N-terminal transmembrane region (residues 1−45) and nine C-terminal residues (202−210); in addition, it contains the point mutation W142F. The sequence contains four histidine residues: two of these (His134 and His154, shown in bold) each ligates an iron atom in the Fe−S cluster; the other two (His120 and His162) are not involved in Fe−S cluster interactions. Residues identified by X-ray crystallography as hydrogen bond donors to the iron sulfur cluster are underlined. Residues with NMR assignments in both oxidized and reduced forms of the protein are highlighted in green. (B) Cross-eyed stereoscopic view of the Fe−S cluster of TtRp and surrounding amino acid residues from the X-ray structure.3 Blue dashed lines indicate hydrogen bonds to the cluster.