Figure 9. Example of an entropic clock. Top panel.
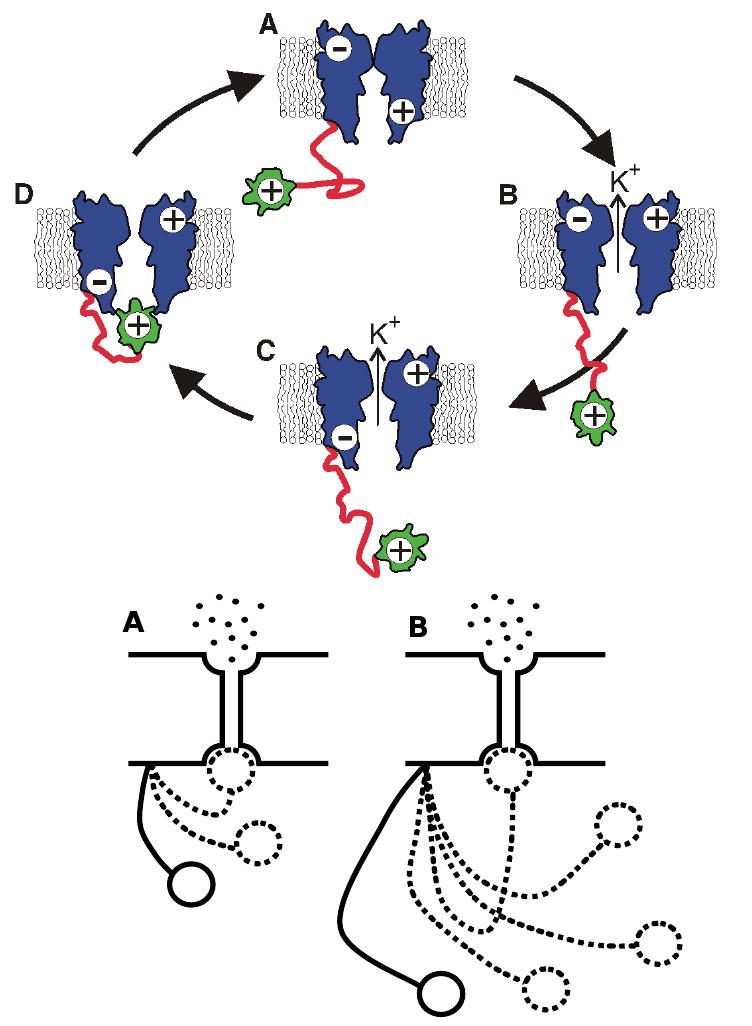
Simplified model of a Shaker-type voltage-gated K+ ion channel (blue) with ‘ball and chain’ timing mechanism. The ‘ball and chain’ is comprised of an inactivation, or ball, domain (yellow) that is tethered to the pore assembly by a disordered chain (red) of ∼ 60 residues. For simplicity, only four of the proposed ten states are shown. The cytoplasmic side of the assembly is oriented downward. A. Closed state prior to membrane depolarization. Note that conformational changes of the pore have sealed the channel and a positive charge on the cytoplasmic side of the pore assembly excludes binding of the ball domain. B. Open state following membrane depolarization. C. After depolarization, the cytoplasmic side of the pore opening assumes a negative charge that facilitates interaction with the positively charged ball domain. D. Inactivation of the channel occurs when the ball domain occludes the pore. The transition from C to D does not involve charge migration and can be modeled as a random walk of the ball domain towards the pore opening. (Portions of figure based on Antz et al. [218]). Bottom panel: Schematic presentation of the ‘chain’ length-dependent timing of channel inactivation. Different lengths of the ‘chain’ region of N-terminal domain result in different rates of channel inactivation [220, 221], where shorter ‘chain’ causes a more rapid inactivation (A), whereas a longer ‘chain’ produces slower inactivation (B). Modified from [225].
