Abstract
Objective:
To characterize weight change during short- and longer-term treatment with desvenlafaxine (administered as desvenlafaxine succinate) for major depressive disorder (MDD).
Method:
Data from 9 short-term, double-blind, placebo-controlled studies and 1 longer-term relapse-prevention trial conducted between September 2002 and January 2007 were analyzed. Adult outpatients with a primary diagnosis of MDD using criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition received fixed- or flexible-dose desvenlafaxine or placebo for 8 weeks in the short-term studies. In the longer-term study, responders to 12 weeks of open-label desvenlafaxine treatment were randomly assigned to double-blind treatment with desvenlafaxine or placebo for 6 months. Mean weight changes and incidence of potentially clinically important changes were evaluated.
Results:
In the short-term studies (desvenlafaxine: n = 1,834; placebo: n = 1,116), mean decreases in weight associated with desvenlafaxine were small but statistically significant compared with baseline (P < .05) and with placebo (final evaluation: –0.82 kg desvenlafaxine vs + 0.05 kg placebo; P < .001). Likewise, during the 12-week, open-label phase of the relapse-prevention study (n = 594), a small but statistically significant mean decrease in weight from baseline (–0.8 kg; P < .001) occurred. Small mean increases in weight (< 1 kg) were observed with both desvenlafaxine (n = 190) and placebo (n = 185) throughout the relapse-prevention phase, with no statistical difference between desvenlafaxine- and placebo-treated patients at the final evaluation. Less than 1% of desvenlafaxine-treated patients experienced a clinically meaningful weight change.
Conclusions:
Desvenlafaxine was not associated with clinically significant weight change during short- or longer-term treatment.
Major depressive disorder (MDD) is often associated with appetite changes (loss of appetite or increased appetite) and subsequent weight changes.1 It is unclear whether weight gain in some depressed patients treated with antidepressant medication is a result of (1) recovery of weight following an improvement in depression symptoms; (2) a residual symptom (ie, in patients who overeat when depressed); or (3) a side effect of the antidepressant itself.1 This ambiguity stems from a methodological issue common to most randomized clinical trials, in which weight data are reported in the aggregate without a clear indication of the potential reasons for weight gain in individual patients.1 Although weight gain is an important factor in patient adherence to medication regimens, weight loss is an important clinical factor in older patients. Depression is the sixth most commonly occurring diagnosis in nursing home residents, occurring in at least 20% of patients.2 Low body weight and weight loss are significant clinical and regulatory issues in nursing facilities and can complicate the course of depression. Therefore, clinicians must consider potential beneficial or detrimental effects on appetite and weight when choosing among available antidepressants for depression in the geriatric population.2
It is clear that monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) exert a pharmacologic effect resulting in weight gain, with reported gains of up to 9 to 18 kg or more for patients treated with MAOIs3 and approximately 2 to 4 kg for those treated with TCAs.4,5 Less clear, however, is the relationship between selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) and weight gain, especially over the long term.1 Patients administered SSRIs tend to lose weight during acute treatment but tend to gain weight over longer periods of treatment.6 Studies have suggested that weight gain is less likely to occur when SSRIs are used for a relatively short time (3–6 months).7 In a comparison of 3 SSRIs—sertraline, fluoxetine, and paroxetine—Fava et al8 reported significant weight gain (> 7%) in 4.2%, 6.8%, and 25.5% of patients, respectively, over a 6-month period. Studies suggest that the SNRI venlafaxine extended release (ER) is no more likely than SSRIs to cause weight gain in the short term,1,9 and that significant weight changes are no more common with maintenance treatment with venlafaxine ER (up to 2 years) than with placebo.10 Patients often need to take antidepressants over the long term; hence it is important that research provide data assessing the impact of long-term treatment with SSRIs or SNRIs on weight.
Clinical Points
♦ Short-term treatment with desvenlafaxine was associated with a small but statistically significant mean decrease in weight (< 1 kg) compared with placebo.
♦ Weight change during longer-term treatment did not differ significantly compared with placebo.
♦ Desvenlafaxine was not associated with clinically significant weight change during short- or longer-term treatment.
Desvenlafaxine (administered as desvenlafaxine succinate) is the major active metabolite of the antidepressant venlafaxine.11 Preclinical studies have demonstrated that desvenlafaxine, like venlafaxine, is an SNRI.12 Clinical studies have demonstrated the safety, efficacy, and tolerability of desvenlafaxine in short- and long-term treatment of MDD.13–21 To examine weight changes in patients with MDD treated with desvenlafaxine in both short- and longer-term treatment, pooled data from 9 placebo-controlled, short-term clinical trials and data from 1 long-term, placebo-controlled, relapse-prevention study were examined.
METHOD
This analysis includes data from 9 short-term studies13,14,16–18,20–22 conducted at 154 sites and 1 relapse-prevention study19 conducted at 49 sites, worldwide. The studies were conducted between September 2002 and January 2007 and in accordance with the ethical principles in the Declaration of Helsinki. Each study protocol and amendments received approval from an institutional review board, independent ethics committee, or both. Written informed consent was obtained from all participants before their enrollment.
Patients
Participants were adult outpatients (≥ 18 years old) with a primary diagnosis of MDD based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition23 criteria, single or recurrent episode, without psychotic features, for at least 30 days and minimum screening and baseline total scores of ≥ 20 (7 studies) or ≥ 22 (2 studies) on the 17-item Hamilton Depression Rating Scale (HDRS17)24 or a score of ≥ 24 on the Montgomery Asberg Depression Rating Scale25 (1 study). Primary exclusion criteria included treatment with desvenlafaxine (at any time) or venlafaxine (within 90 days); known hypersensitivity to venlafaxine; significant risk of suicide; women who were pregnant, breastfeeding, or planning to become pregnant during the study; current (within 12 months before baseline) psychoactive substance abuse or dependence (including alcohol); manic episode, anxiety disorder, or a lifetime diagnosis of bipolar or psychotic disorder; major acute illness during the 90 days before screening; or clinically important abnormalities on physical examination or electrocardiogram and laboratory tests.
Treatment
The 9 short-term desvenlafaxine studies13,14,16–18,20–22 were multicenter, randomized, double-blind, parallel-group studies in which adult outpatients with MDD received desvenlafaxine (n = 1,834) or placebo (n = 1,116) for 8 weeks, followed by a taper period of up to 2 weeks (Table 1). Four were flexible-dose studies: 100 to 200 mg/d (1 study) and 200 to 400 mg/d (3 studies); and 5 were fixed-dose studies: 50 or 100 mg/d (2 studies), 200 or 400 mg/d (2 studies), or 100, 200, or 400 mg/d (1 study). In the relapse-prevention study,19 patients initially received 12 weeks of open-label desvenlafaxine treatment (flexible dose of 200 to 400 mg/d; n = 594). Responders (HDRS17 score ≤ 11 at end of week 12; n = 375) were randomly assigned to double-blind treatment with desvenlafaxine (n = 190) or placebo (n = 185) for 6 months (Table 1).
Table 1.
Number of Subjects and Doses in 10 Desvenlafaxine Studies of Patients With MDD (safety population)
| Desvenlafaxine |
||||
| Type of Study | Dose, mg/d | Mean Daily Dose After Titration, mg (completers) | n | Placebo, n |
| Short term (8 wk), double-blind | ||||
| Fixed dose | ||||
| Study 223a | 200 or 400 | … | 144 | 78 |
| Study 306b | 100, 200, or 400 | … | 350 | 120 |
| Study 308c | 200 or 400 | … | 248 | 125 |
| Study 332d | 50 or 100 | … | 299 | 152 |
| Study 333e | 50 or 100 | … | 324 | 161 |
| Flexible dose | ||||
| Study 304f | 100–200 | 182.4–195.2 | 121 | 117 |
| Study 309g | 200–400 | 197.6–301.9 | 117 | 120 |
| Study 317g | 200–400 | 194.7–336.1 | 114 | 125 |
| Study 320h | 200–400 | 333.8–376.8 | 117 | 118 |
| Total | … | … | 1,834 | 1,116 |
| Relapse prevention | ||||
| Study 302i | ||||
| Open-label, 12 wk | 200–400 | … | 594 | … |
| Double-blind, 6 mo | 200–400 | … | 190 | 185 |
Wyeth Pharmaceuticals.22
DeMartinis et al.13
Septien-Velez et al.14
Liebowitz et al.16
Boyer et al.21
Liebowitz et al.17
Lieberman et al.18
Feiger et al.20
Rickels et al19; after 12 weeks of open-label treatment with desvenlafaxine, responders were randomly assigned to receive 6 months of double-blind treatment with desvenlafaxine or placebo.
Abbreviation: MDD = major depressive disorder.
Symbol: … = not applicable.
Assessments and Statistical Analysis
Weight was assessed at baseline and weeks 1, 2, 3, 4, 6, and 8 in the short-term studies and at baseline and weeks 1, 2, 3, 4, 8, 11, and 12 of open-label treatment and weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24 of double-blind treatment in the relapse-prevention study. In addition to observed data from each visit, data from the final evaluation (ie, data from all patients, using the last assessment prior to discontinuation) were analyzed. Analyses were performed on data from the safety population, which included all patients who received at least 1 dose of study medication. Weight comparisons between placebo and desvenlafaxine treatments were based on adjusted mean changes from baseline (ie, the measurement closest to the start of the on-therapy period of acute-phase treatment), using analysis of covariance with baseline as covariate. Statistically significant changes from baseline and significant differences between groups were declared at P ≤ .05. The definition of potentially clinically important (PCI) weight change was based on the established United States Food and Drug Administration metric of an increase or decrease from baseline of 7% or more. Data from patients with weight changes meeting the PCI criterion were reviewed prior to unblinding to assess the clinical meaningfulness of that weight change, judged subjectively as the amount of change that a patient would find noticeable or bothersome.
RESULTS
The population among the 9 short-term studies and the relapse-prevention study was predominantly white and female, with a mean age and weight similar across all groups. Table 2 outlines the demographic and baseline characteristics of the safety population for all short-term studies and the open-label and double-blind phases of the relapse-prevention study.
Table 2.
Demographic and Baseline Characteristics of Patients With MDD Treated With Desvenlafaxine (safety population)
| Relapse Prevention Studyb |
|||||
| Short-Term, All Studiesa |
Open-Label Phase, Desvenlafaxine, 200–400 mg | Double-Blind Phase |
|||
| Characteristic | Placebo | Desvenlafaxine, 50–400 mg | Placebo | Desvenlafaxine, 200–400 mg | |
| Safety population, n | 1,116 | 1,834 | 594 | 185 | 190 |
| ITT population, n | 1,108 | 1,805 | 575 | 185 | 189 |
| Age, mean (SD), y | 42.4 (12.7) | 42.5 (12.6) | 41.9 (12.6) | 42.8 (11.8) | 42.7 (12.3) |
| Weight, mean (SD), kg | 79.0 (19.7)c | 79.9 (20.0) | 77.5 (19.3) | 76.8 (18.7) | 78.7 (19.6) |
| Ethnicity, n (%)d | |||||
| Asian | 10 (1) | 20 (1) | 12 (2) | 4 (2) | 1 (1) |
| Black | 105 (9) | 156 (9) | 36 (6) | 8 (4) | 11 (6) |
| Hispanic | 66 (6) | 121 (7) | 33 (6) | 7 (4) | 7 (4) |
| Other | 21 (2) | 25 (1) | 5 (1) | 2 (1) | 2 (1) |
| White | 909 (81) | 1,504 (82) | 503 (85) | 161 (87) | 169 (89) |
| Sex, n (%) | |||||
| Female | 713 (64) | 1,111 (61) | 404 (68) | 126 (68) | 127 (67) |
| Male | 403 (36) | 723 (39) | 190 (32) | 59 (32) | 63 (33) |
DeMartinis et al,13 Septien-Velez et al,14 Liebowitz et al,16 Liebowitz et al,17 Lieberman et al,18 Feiger et al,20 Boyer et al,21 and Wyeth Pharmaceuticals.22
Rickels et al.19
n = 1,115.
Percent totals may not equal 100 due to rounding.
Abbreviations: ITT = intent-to-treat; SD = standard deviation.
Individual Weight Changes
In the short-term studies, the incidence of PCI weight changes was approximately 3% among desvenlafaxine-treated patients (0.8% weight gain, 2.1% weight loss) and 2% in the placebo group (1.0% weight gain, 0.7% weight loss; Table 3). In the relapse-prevention study, the incidence of PCI weight changes during open-label desvenlafaxine treatment was 10% (2.6% weight gain, 6.9% weight loss). The incidence of PCI weight changes during the double-blind phase was 31% for desvenlafaxine (19.1% weight gain, 12.2% weight loss) and 19% for placebo (12.0% weight gain, 7.1% weight loss; Table 3).
Table 3.
Number (%) of MDD Subjects With Potentially Clinically Important Weight Change (≥ 7% from baseline)
| Short-Term Studies |
Relapse-Prevention Studyc |
|||||||||
| All Studiesa |
Fixed Doseb |
Open-Label Phase, Desvenlafaxine 200–400 mg (n = 548) | Double-Blind Phase |
|||||||
| Desvenlafaxine, 50–400 mg (n = 1,733) | Desvenlafaxine |
|||||||||
| Weight Change | Placebo (n = 1,097) | Placebo (n = 623) | 50 mg (n = 309) | 100 mg (n = 410) | 200 mg (n = 282) | 400 mg (n = 300) | Placebo (n = 184) | Desvenlafaxine 200–400 mg (n = 188) | ||
| Weight gain | 11 (1.0) | 14 (0.8) | 5 (0.8) | 3 (1.0) | 3 (0.7) | 3 (1.1) | 1 (0.3) | 14 (2.6) | 22 (12.0) | 36 (19.1) |
| Weight loss | 8 (0.7) | 37 (2.1) | 5 (0.8) | 5 (1.6) | 10 (2.4) | 2 (0.7) | 11 (3.7) | 38 (6.9) | 13 (7.1) | 23 (12.2) |
DeMartinis et al,13 Septien-Velez et al,14 Liebowitz et al,16 Liebowitz et al,17 Lieberman et al,18 Feiger et al,20 Boyer et al,21 and Wyeth Pharmaceuticals.22
DeMartinis et al,13 Septien-Velez et al,14 Liebowitz et al,16 Boyer et al,21 and Wyeth Pharmaceuticals.22
Rickels et al.19
Abbreviation: MDD = major depressive disorder.
Before the treatment data were unblinded, the sponsor's medical monitor reviewed the records and correspondence of all patients with weight changes meeting PCI criteria to assess the clinical meaningfulness of the changes. In the short-term studies, 2 women from the desvenlafaxine group and 1 man from the placebo group had clinically meaningful weight gain (5.8 kg, 5.1 kg, and 6.5 kg, respectively). Three women from the desvenlafaxine group and none in the placebo group had clinically meaningful weight loss (5 kg, 5.9 kg, and 10 kg, respectively). In the relapse-prevention study, 1 man treated with desvenlafaxine had clinically meaningful weight gain in both the open-label and double-blind phases (up to 13.6 kg after 20 weeks of treatment; 11.4 kg over baseline at the end of the 9-month study). One woman randomly assigned to placebo for the double-blind phase had clinically meaningful weight gain during the desvenlafaxine open-label phase (6.6 kg) and gained an additional 0.2 kg during the 2-week taper from desvenlafaxine to placebo before she discontinued because of unsatisfactory response.
Mean Weight Changes
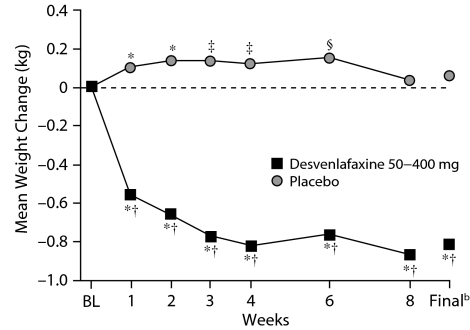
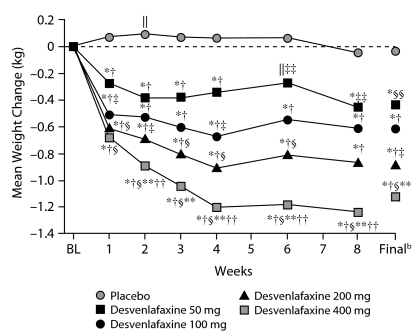
In the 9 short-term studies, desvenlafaxine treatment was associated with small but statistically significant mean decreases in weight from baseline throughout the treatment period (P < .001). Placebo was associated with small mean increases in weight that were statistically significant at weeks 1 through 6 (P ≤ .05). Differences between desvenlafaxine and placebo were significant at all weeks (P < .001) and at the final evaluation (–0.82 kg desvenlafaxine vs +.05 kg placebo, P < .001; Figure 1). In the 5 fixed-dose studies, all doses of desvenlafaxine were associated with modest (0.26 kg to 1.26 kg) but statistically significant (P ≤ .05) mean weight loss throughout the treatment period, whereas placebo was associated with small mean weight gain (< 0.15 kg) at weeks 1 through 6, followed by mean weight loss (0.04 kg) at week 8. Differences between desvenlafaxine and placebo were significant at all weeks and at the final evaluation (Figure 2). Mean weight loss for the 200 mg/d group was significantly greater compared with the 50 mg/d group, and the 400 mg/d group differed significantly from both the 50 mg/d and 100 mg/d groups.
Figure 1.
Mean Weight Change (kg) From Baseline Over Time in 9 Short-Term, Double-Blind, Placebo-Controlled Studies of Patients With MDDa
aPooled data from 9 short-term studies.13,14,16–18,20–22
bFinal refers to the final evaluation and includes data from all patients using the last assessment prior to discontinuation.
*P < .001 for within-group change from baseline.
†P < .001 desvenlafaxine versus placebo.
‡P < .01 for within-group change from baseline.
§P < .05 for within-group change from baseline.
Abbreviations: BL = baseline, MDD = major depressive disorder.
Figure 2.
Mean Weight Change (kg) From Baseline Over Time in 5 Short-Term, Double-Blind, Placebo-Controlled Fixed-Dose Studies of Desvenlafaxine in Patients With MDDa
aData from 5 short-term, fixed-dose studies.13,14,16,21,22
bFinal refers to the final evaluation and includes data from all patients using the last assessment prior to discontinuation.
*P < .001 within-group change from baseline.
†P < .001 desvenlafaxine versus placebo.
‡P < .01 versus desvenlafaxine 50 mg.
§P ≤ .001 versus desvenlafaxine 50 mg.
‖P < .05 within-group change from baseline.
**P < .001 versus desvenlafaxine 100 mg.
††P < .05 versus desvenlafaxine 200 mg.
‡‡P < .05 desvenlafaxine versus placebo.
§§P < .01 desvenlafaxine versus placebo.
Abbreviations: BL = baseline, MDD = major depressive disorder.
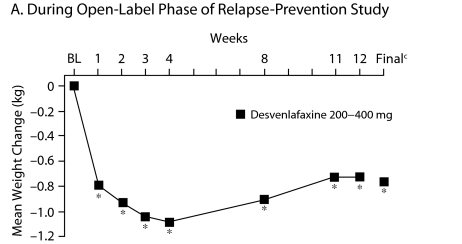
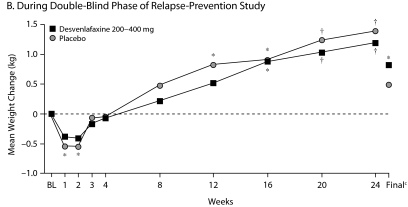
In the open-label phase of the relapse-prevention study, mean weight loss at the final evaluation was < 1 kg (P ≤ .001 vs baseline, Figure 3A). During the double-blind phase, there was a weight gain in both the placebo and desvenlafaxine groups. Mean weight change from baseline (ie, start of open-label phase) during the double-blind phase of the relapse-prevention trial was significant for desvenlafaxine at weeks 16, 20, 24, and at the final evaluation (P ≤ .05). Changes from baseline in the placebo group were significant at weeks 1, 2, 12, 16, 20, and 24 (P ≤ .05). Differences between desvenlafaxine and placebo during double-blind treatment were not significant at any week or at the final evaluation (Figure 3B).
Figure 3.
Mean Weight Change (kg) From Baselinea Over Time in Patients With MDDb
aBaseline refers to start of open-label treatment.
bRickels et al.19
cFinal refers to the final evaluation and includes data from all patients using the last assessment prior to discontinuation.
*P = .001 for within-group change from baseline.
†P ≤ .05 for within-group change from baseline.
‡P < .01 for within-group change from baseline.
Abbreviations: BL = baseline, MDD = major depressive disorder.
DISCUSSION
Overall, short-term treatment with desvenlafaxine was associated with small mean decreases in weight (< 1 kg) that were statistically significant compared with baseline and compared with the small mean increase (< 1 kg) associated with placebo (P < .001). The results suggest that weight loss associated with short-term desvenlafaxine treatment may increase slightly with higher doses. Longer-term treatment (up to 9 months) with desvenlafaxine or placebo was associated with a small but statistically significant mean increase (< 1 kg) in weight from baseline; however, the difference between groups was not significant. It is not known whether a longer duration of treatment with desvenlafaxine might result in further weight gain. Mean weight loss seen in the open-label phase of the relapse-prevention study was similar in magnitude to that seen in the short-term studies, suggesting a consistency in response. Only 7 out of 2,469 desvenlafaxine patients and 1 out of 1,281 placebo patients experienced weight changes that were judged to be clinically meaningful.
Weight changes occurring during the early weeks of treatment will not necessarily continue at the same rate throughout treatment, yielding larger cumulative changes over time. SSRIs tend to induce weight loss during acute treatment followed by weight gain during long-term treatment.6 The small mean weight loss in this analysis of short-term studies and the small mean weight gain found in the relapse prevention study are consistent with findings for SSRIs and for the SNRI duloxetine.6,26,27 Early weight loss in the current analysis may be related to transient nausea, the most common adverse event associated with short-term desvenlafaxine treatment.13,14,17,18 However, incidence of nausea is highest during the first week of desvenlafaxine treatment and declines to placebo levels after that.13,14,17,18 In the current analysis, weight loss continued through at least the first 12 weeks of treatment.
Weight gain is a reported side effect of long-term antidepressant treatment with MAOIs, TCAs, and some SSRIs.3–6,8,28,29 Possible mechanisms underlying antidepressant-associated weight gain include changes in dopamine and serotonin concentrations and to their postsynaptic receptors in response to food ingestion,30 blockade of histamine H1 and serotonin 2C receptors, carbohydrate craving caused by α-noradrenergic activity or histamine blockade, or changes in the regulation of body fat stores by modulating neurotransmitter systems at the hypothalamic level.1 Weight gain during antidepressant treatment may also reflect an improvement in symptoms of depression. In one study,31 72% of remitted patients with bipolar or unipolar depression exhibited weight gain (mean: 6.43 kg) compared with their pretreatment baseline, and weight gain at remission was associated with weight loss during depression. Although the study did not compare remitted patients who were treated with antidepressants versus patients who remitted during nonpharmacologic treatment, the authors suggested that weight gain in remitted depressed patients may be a sign of recovery from depression rather than a pharmacologic effect of the antidepressants.31 A study showing that the frequency distribution of body weight for inpatients treated for MDD32 was skewed toward lower weight at admission and moved toward a more normal distribution after treatment with TCAs supports this view.
Although the average weight change with desvenlafaxine treatment was less than 1 kg, 3% of patients in the short-term studies (placebo, 2%) and 31% in the longer duration study (placebo, 19%) had potentially clinically important weight change (≥ 7% from baseline). The clinical significance of weight losses or gains may vary for different patient populations. On average, early weight loss subsides after the first few weeks of desvenlafaxine treatment and may be easily managed with patient education or other pharmacologic intervention.33 Clinicians may pay particular attention to weight loss in elderly patients, however. Studies of older adults in the community show that weight loss of 4%–5% of body weight over 1 to 3 years is associated with an approximately 2-fold higher risk of mortality.34,35 Moderate weight gain may also be clinically meaningful to certain patient populations: weight gain can adversely affect compliance,33,36,37 and women, for example, may be particularly likely to have concerns about weight gain.38,39
Limitations of this analysis include the significant exclusionary criteria that presumably limit generalizability to typical outpatients and the lack of a method to assess causal factors related to weight changes, although these design characteristics are not unique to this particular group of studies. Nonetheless, the results of this analysis, in an apparently representative cohort of depressed, ambulatory patients, provide useful and clinically relevant information. The analysis was also limited by the relatively small amount of long-term data. A large majority of patients require long-term treatment in order to achieve and sustain remission, and treatment guidelines recommend that clinicians consider maintenance-phase treatment for patients with multiple episodes of MDD or those who show persistence of dysthymic symptoms.40 Additional data are thus needed to determine whether desvenlafaxine may be associated with clinically significant weight gain with treatment durations of a year or more and whether weight gain during treatment might be related to baseline weight in patients with MDD.
Accumulating data suggest that weight change, particularly weight gain, is a class effect of TCAs and MAOIs and may also be an issue for patients treated with some SSRIs. The impact of weight changes on treatment compliance should be considered with all patients who are treated with antidepressants during both acute and long-term treatment. Desvenlafaxine represents a novel antidepressant that is not likely to be associated with clinically significant weight change during short- or longer-term treatment.
Drug names: desvenlafaxine (Pristiq), duloxetine (Cymbalta), fluoxetine (Prozac and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others), venlafaxine (Effexor and others).
Potential conflicts of interest: Dr Tourian is an employee and stock shareholder of Pfizer, Inc, formerly Wyeth Research. Dr Leurent is an employee of Wyeth, a company of the Pfizer Group. Drs Graepel and Ninan are employees of Pfizer Inc, formerly Wyeth Research.
Funding/support: The clinical trials and analysis were sponsored by Wyeth Research, Collegeville, Pennsylvania, which was acquired by Pfizer Inc in October 2009.
Acknowledgments
This study was sponsored by Wyeth Research, which was acquired by Pfizer Inc in October 2009. Medical writing support for this manuscript was provided by Kathleen M. Dorries, PhD, of Advogent, and was funded by Wyeth.
REFERENCES
- 1.Fava M. Weight gain and antidepressants. J Clin Psychiatry. 2000;61(suppl 11):37–41. [PubMed] [Google Scholar]
- 2.Rigler SK, Webb MJ, Redford L, et al. Weight outcomes among antidepressant users in nursing facilities. J Am Geriatr Soc. 2001;49(1):49–55. doi: 10.1046/j.1532-5415.2001.49009.x. [DOI] [PubMed] [Google Scholar]
- 3.Cantu TG, Korek JS. Monoamine oxidase inhibitors and weight gain. Drug Intell Clin Pharm. 1988;22(10):755–759. doi: 10.1177/106002808802201002. [DOI] [PubMed] [Google Scholar]
- 4.Garland EJ, Remick RA, Zis AP. Weight gain with antidepressants and lithium. J Clin Psychopharmacol. 1988;8(5):323–330. [PubMed] [Google Scholar]
- 5.Fernstrom MH, Kupfer DJ. Antidepressant-induced weight gain: a comparison study of four medications. Psychiatry Res. 1988;26(3):265–271. doi: 10.1016/0165-1781(88)90120-5. [DOI] [PubMed] [Google Scholar]
- 6.Hirschfeld RM. Long-term side effects of SSRIs: sexual dysfunction and weight gain. J Clin Psychiatry. 2003;64(suppl 18):20–24. [PubMed] [Google Scholar]
- 7.Deshmukh R, Franco K. Managing weight gain as a side effect of antidepressant therapy. Cleve Clin J Med. 2003;70614(7) doi: 10.3949/ccjm.70.7.614. 616, 618, passim. [DOI] [PubMed] [Google Scholar]
- 8.Fava M, Judge R, Hoog SL, et al. Fluoxetine versus sertraline and paroxetine in major depressive disorder: changes in weight with long-term treatment. J Clin Psychiatry. 2000;61(11):863–867. doi: 10.4088/jcp.v61n1109. [DOI] [PubMed] [Google Scholar]
- 9.Silverstone PH, Ravindran A. Once-daily venlafaxine extended release (XR) compared with fluoxetine in outpatients with depression and anxiety. Venlafaxine XR 360 Study Group. J Clin Psychiatry. 1999;60:22–28. doi: 10.4088/jcp.v60n0105. [DOI] [PubMed] [Google Scholar]
- 10.Keller MB, Trivedi MH, Thase ME, et al. The prevention of recurrent episodes of depression with venlafaxine for two years (PREVENT) study: outcomes from the 2-year and combined maintenance phases. J Clin Psychiatry. 2007;68(8):1246–1256. doi: 10.4088/jcp.v68n0812. [DOI] [PubMed] [Google Scholar]
- 11.Johnston G, Bray J, Andree T. Annual Meeting of the Society for Neuroscience; Washington, DC: Desvenlafaxine succinate: a new serotonin and norepinephrine reuptake inhibitor [poster] Presented at. November 3–5, 2005. [Google Scholar]
- 12.Parks V, Patat A, Behrle J, et al. Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics Annual Meeting; Orlando, Florida: Safety, pharmacokinetics (PK) and pharmacodynamics (PD) of ascending single doses of desvenlafaxine (DVS-233 SR) in healthy subjects [poster]. Presented at. March 2–6, 2005. [Google Scholar]
- 13.DeMartinis NA, Yeung PP, Entsuah R, et al. A double-blind, placebo-controlled study of the efficacy and safety of desvenlafaxine succinate in the treatment of major depressive disorder. J Clin Psychiatry. 2007;68(5):677–688. doi: 10.4088/jcp.v68n0504. [DOI] [PubMed] [Google Scholar]
- 14.Septien-Velez L, Pitrosky B, Padmanabhan SK, et al. A randomized, double-blind, placebo-controlled trial of desvenlafaxine succinate in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2007;22(6):338–347. doi: 10.1097/YIC.0b013e3281e2c84b. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson J, Tourian KA, Rosas GR, et al. Annual Meeting of the American Psychiatric Association; San Diego, California: A 12-month open-label evaluation of long-term safety and efficacy of desvenlafaxine succinate in outpatients with major depressive disorder [poster] Presented at. May 19–24, 2007. [Google Scholar]
- 16.Liebowitz MR, Manley AL, Padmanabhan SK, et al. Efficacy, safety, and tolerability of desvenlafaxine 50 mg/day and 100 mg/day in outpatients with major depressive disorder. Curr Med Res Opin. 2008;24(7):1877–1890. doi: 10.1185/03007990802161923. [DOI] [PubMed] [Google Scholar]
- 17.Liebowitz MR, Yeung PP, Entsuah R. A randomized, double-blind, placebo-controlled trial of desvenlafaxine succinate in adult outpatients with major depressive disorder. J Clin Psychiatry. 2007;68(11):1663–1672. doi: 10.4088/jcp.v68n1105. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman DZ, Montgomery SA, Tourian KA, et al. A pooled analysis of two placebo-controlled trials of desvenlafaxine in major depressive disorder. Int Clin Psychopharmacol. 2008;23(4):188–197. doi: 10.1097/YIC.0b013e32830263de. [DOI] [PubMed] [Google Scholar]
- 19.Rickels K, Montgomery SA, Tourian KA, et al. Desvenlafaxine for the prevention of relapse in major depressive disorder: results of a randomized trial. J Clin Psychopharmacol. 2010;30(1):18–24. doi: 10.1097/JCP.0b013e3181c94c4d. [DOI] [PubMed] [Google Scholar]
- 20.Feiger AD, Tourian K, Rosas G, et al. A placebo-controlled efficacy and safety study of a flexible dose of desvenlafaxine in outpatients with major depressive disorder. CNS Spectr. 2009;14(1):41–50. doi: 10.1017/s1092852900020046. [DOI] [PubMed] [Google Scholar]
- 21.Boyer P, Montgomery S, Lepola U, et al. Efficacy, safety, and tolerability of fixed-dose desvenlafaxine 50 and 100 mg/day for major depressive disorder in a placebo-controlled trial. Int Clin Psychopharmacol. 2008;23(5):243–253. doi: 10.1097/YIC.0b013e32830cebed. [DOI] [PubMed] [Google Scholar]
- 22.Wyeth Pharmaceuticals. Wyeth Pharmaceuticals Study 223. http://www.clinicalstudyresults.org/documents/company-study_8588_0.pdf Accessed January 4, 2010.
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 26.Raskin J, Goldstein DJ, Mallinckrodt CH, et al. Duloxetine in the long-term treatment of major depressive disorder. J Clin Psychiatry. 2003;64(10):1237–1244. doi: 10.4088/jcp.v64n1015. [DOI] [PubMed] [Google Scholar]
- 27.Dunner DL. Venlafaxine in the treatment of unresolved symptoms of depression following antidepressant therapy. Prim Psychiatry. 2007;14(3):39–49. [Google Scholar]
- 28.Cassano P, Fava M. Tolerability issues during long-term treatment with antidepressants. Ann Clin Psychiatry. 2004;16(1):5–25. doi: 10.1080/10401230490281618. [DOI] [PubMed] [Google Scholar]
- 29.Harvey BH, Bouwer CD. Neuropharmacology of paradoxic weight gain with selective serotonin reuptake inhibitors. Clin Neuropharmacol. 2000;23(2):90–97. doi: 10.1097/00002826-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Meguid MM, Fetissov SO, Varma M. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16(10):843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 31.Benazzi F. Weight gain in depression remitted with antidepressants: pharmacological or recovery effect? Psychother Psychosom. 1998;67(4-5):271–274. doi: 10.1159/000012291. [DOI] [PubMed] [Google Scholar]
- 32.Shioiri T, Kato T, Murashita J, et al. Changes in the frequency distribution pattern of body weight in patients with major depression. Acta Psychiatr Scand. 1993;88(5):356–360. doi: 10.1111/j.1600-0447.1993.tb03472.x. [DOI] [PubMed] [Google Scholar]
- 33.Zajecka JM. Clinical issues in long-term treatment with antidepressants. J Clin Psychiatry. 2000;61(suppl 2):20–25. [PubMed] [Google Scholar]
- 34.Newman AB, Yanez D, Harris T, et al. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49(10):1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 35.Wallace JI, Schwartz RS, LaCroix AZ, et al. Involuntary weight loss in older outpatients: incidence and clinical significance. J Am Geriatr Soc. 1995;43(4):329–337. doi: 10.1111/j.1532-5415.1995.tb05803.x. [DOI] [PubMed] [Google Scholar]
- 36.Lin EH, Von Korff M, Katon W, et al. The role of the primary care physician in patients’ adherence to antidepressant therapy. Med Care. 1995;33(1):67–74. doi: 10.1097/00005650-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Berken GH, Weinstein DO, Stern WC. Weight gain. A side-effect of tricyclic antidepressants. J Affect Disord. 1984;7(2):133–138. doi: 10.1016/0165-0327(84)90031-4. [DOI] [PubMed] [Google Scholar]
- 38.Cash TF, Brown MA. Attitudes about antidepressants: influence of information about weight-related side effects. Percept Mot Skills. 2000;90(2):453–456. doi: 10.2466/pms.2000.90.2.453. [DOI] [PubMed] [Google Scholar]
- 39.Goethe JW, Woolley SB, Cardoni AA, et al. Selective serotonin reuptake inhibitor discontinuation: side effects and other factors that influence medication adherence. J Clin Psychopharmacol. 2007;27(5):451–458. doi: 10.1097/jcp.0b013e31815152a5. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 2nd ed. Arlington, Virginia: American Psychiatric Association; 2000. [PubMed] [Google Scholar]