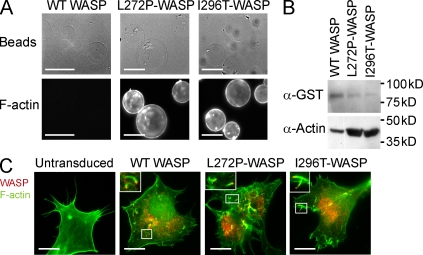
Figure 1.
WASP-L272P and WASP-I296T induce increased actin polymerization in vitro, and are stably expressed and functional in live cells. (A and B) In vitro actin-polymerizing assay. GST-WASP fusion proteins were purified with glutathione sepharose beads and incubated with cell lysates to induce actin polymerization. Polymerized actin (F-actin) was visualized on beads after labeling with phalloidin (A) or by Western blotting using anti-actin antibodies (B). Bars, 100 µm. (C) Expression of WASP and rescue of vaccinia actin tail formation. Retrovirions expressing WASP-WT, WASP-L272P, and WASP-I296T with an N-terminal flag tag were used for protein expression in N-WASP–deficient fibroblasts. The cells were subsequently infected with vaccinia virus that forms characteristic actin tails visualized with phalloidin (green). Localization of WASP proteins was determined using anti-flag antibodies (red). Vaccinia fails to form tails in untransduced N-WASP–deficient fibroblasts (left). Insets show higher magnification of boxed areas highlighting vaccinia virus actin tails. Bars, 20 µm. These experiments represent one of at least three independent experiments.