Fig. 1.
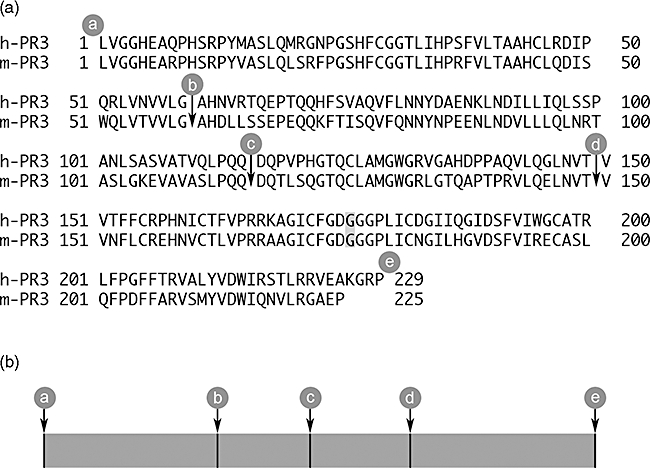
(a) The amino acid sequences for human proteinase 3 (h-PR3) and murine PR3 (m-PR3) used as templates for the recombinant proteins. The signal peptides and propeptides are removed and a HindIII site is introduced at the 5′ end and a NotI site is introduced at the 3′ end. All the recombinant molecules were mutated at the active site by changing Ser to Gly (shaded) to create enzymatically inactive mutants. The cleavage sites for the different constructs are marked a, b, c, d, and e. (b) The chimeric constructs were named according to the origin of the respective proportions of the molecule, were H stands for hPR3 and m for mPR3; for example, mmH is comprised of murine sequence from a to d followed by human sequence from d to e.
