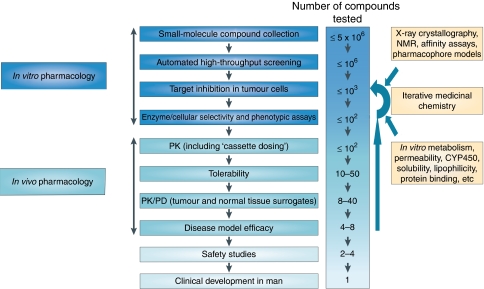
Figure 2.
Example of a drug discovery test cascade for identifying small-molecule antitumour drugs. A representative test cascade for identifying a potential small-molecule drug against a given target is shown. A subset of a compound library is initially screened vs the target in vitro, in recombinant protein or cellular assays, using high-throughput automation to identify ‘hits’. Subsequent leads are examined in more detail by assessing their effect on downstream molecular events in cells and their selectivity vs other proteins. A battery of additional in vitro tests is also used for measurement or prediction of physical properties and pharmacokinetic parameters. Only compounds with a promising balance of features are progressed to in vivo testing, usually in mice. Pharmacokinetic (PK) studies, used to understand drug exposure, may initially involve co-inoculation of low doses of compounds (‘cassette dosing’) to minimise animal usage. The tolerability of leads with favourable PK is then assessed at higher doses, before evaluating their pharmacodynamic (PD) effect on tumour and normal tissues at well-tolerated doses. Compounds that do not meet the anticipated level of performance at any stage may result in subsequent rounds of iterative medicinal chemistry to generate improved leads. Selected leads are progressed to efficacy testing to determine the link between target inhibition and the effect on tumour growth or spread (metastasis). Safety studies on late-stage leads are also required before a candidate drug can be selected for examination in cancer patients (not covered here). The application of the test cascade means that compounds are filtered by the earlier stage assays so that a smaller number of compounds, and only those of higher quality, are taken into later stage in vivo assays in animals.