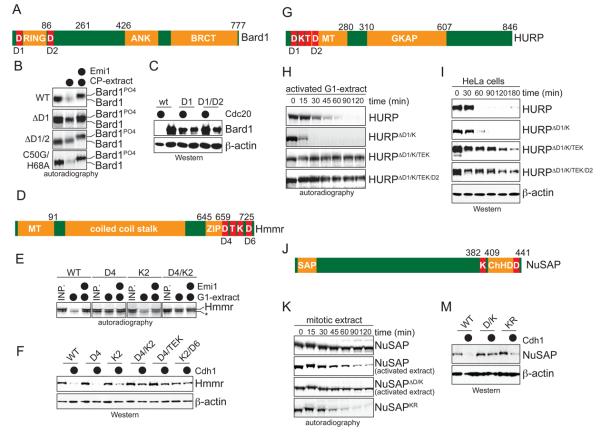
Figure 3. The SAF-degrons are adjacent to domains required for spindle assembly.
A. Schematic overview of Bard1 (RING: RING-domain; ANK: ankyrin repeats; BRCT: Brct-domain; D: D-box). B. Mutation of D-boxes stabilizes Bard1 in mitotic extracts. 35S-labeled mutants were tested for APC/C-dependent degradation in mitotic extracts with active APC/CCdc20. The C50G/H68A-mutant ablates the function of the RING-domain, but has no effect on APC/C-dependent degradation. C. Mutation of D-boxes stabilizes Bard1 in vivo. 293T cells were co-transfected with Bard1-mutants and Cdc20, and Bard1-levels were determined by Western blot. D. Schematic overview of Hmmr. (MT: microtubule-binding domain; ZIP: leucine zipper; T: TEK-box; K: KEN-box) E. Mutation of a D- and KEN-box stabilizes Hmmr in G1-extracts. The indicated 35S-labeled point mutants were tested for APC/C-dependent degradation in G1-extracts as described above. Reaction products were analyzed by autoradiography. F. Mutation of D-, KEN-, and TEK-boxes stabilize Hmmr in vivo. mycHmmr or the indicated mutants were co-expressed in 293T cells with Cdh1, and protein levels were analyzed by Western blot using αmyc-antibodies. G. Schematic overview of HURP. (MT: microtubule binding domain; GKAP: GKAP-domain). H. The degradation of HURP in G1-extracts depends on D-, KEN-, and TEK-boxes. To ensure degradation of wt-HURP, G1-extracts were treated with RanQ69L (see later). The indicated 35S-labeled mutants were incubated in G1-extracts before being analyzed by autoradiography. I. Degradation of HAHURP and its mutants in HeLa cells after release from nocodazole arrest. Samples were taken at the indicated times and analyzed for HURP-levels by Western blot. J. Schematic overview of NuSAP. The SAP- and ChHD-domains were defined by Rijmaekers et al., 2003. K. Degradation of NuSAP in mitotic extracts depends on D- and KEN-boxes. If indicated, the mitotic extract was supplemented with RanGTP. 35S-labeled mutants were incubated in the extracts for different times, before being analyzed by autoradiography. In NuSAPKR, a stretch of Lys residues has been changed to alanines, leading to its degradation in the absence of RanGTP. M. The degradation of NuSAP in cells depends on D- and KEN-boxes. The respective HANuSAP mutants (wt; DK: mutation of D- and KEN-box; KR: mutation of a Lys-rich cluster) were co-expressed with Cdh1 in 293T cells, as indicated. The levels of NuSAP and its mutants were detected by Western blot.