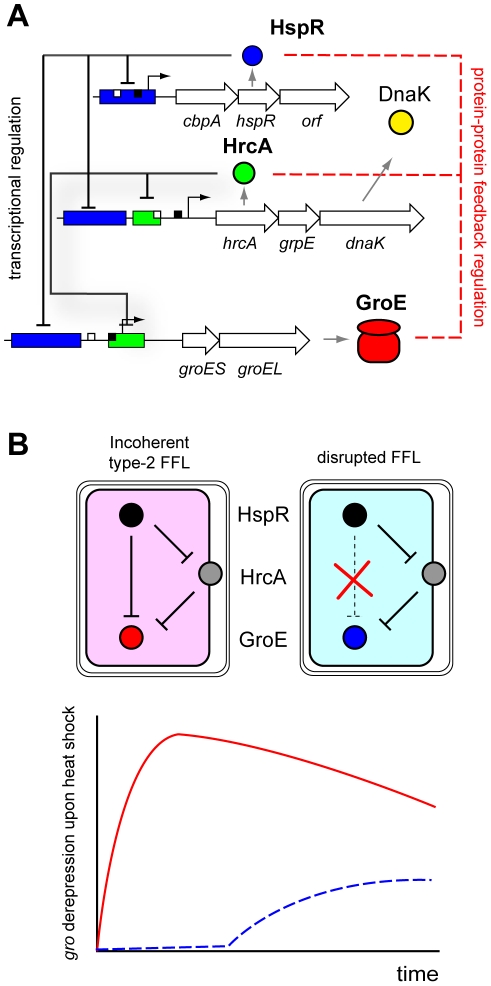
Figure 2. The heat shock and stress response origon.
A. The heat shock origon is composed of two TFs (HspR and HrcA) repressing directly three main target operons: I) the cbp operon, encoding, respectively, a DnaJ-homologue CbpA, the HspR regulator itself, and a gene product of unknown function with homology to a helicase (HP1026); II) the hrcA operon, coding for the HrcA regulator, DnaK, and the GrpE co-chaperone; and III) the groESL operon, encoding conserved GroES and GroEL chaperones. Block arrows: open reading frames. Green boxes: HrcA operators; blue boxes, HspR operators. −10 and −35 boxes are depicted by black and white squares, respectively. TF–DNA interactions and direct transcriptional control are depicted by black lines; red dotted lines represent protein–protein interactions important for feedback control of the circuit; arrowheads denote positive regulation; hammerheads indicate negative regulation. B. Incoherent type-2 FFL wiring of HspR, HrcA, and the groESL operon, responsible for the prompt derepression kinetics of Pgro upon heat shock. Red line: derepression kinetic of the intact FFL motif; blue dotted line: derepression kinetic of a mutated Pgro promoter in which the HspR operator has been deleted. The membrane association of HrcA in H. pylori [47] may confer additional sensory potential to the circuit, e.g., through sensing of periplasmic misfolded peptides, or need to release HrcA from the inner membrane by a stress-inducible co-factor.