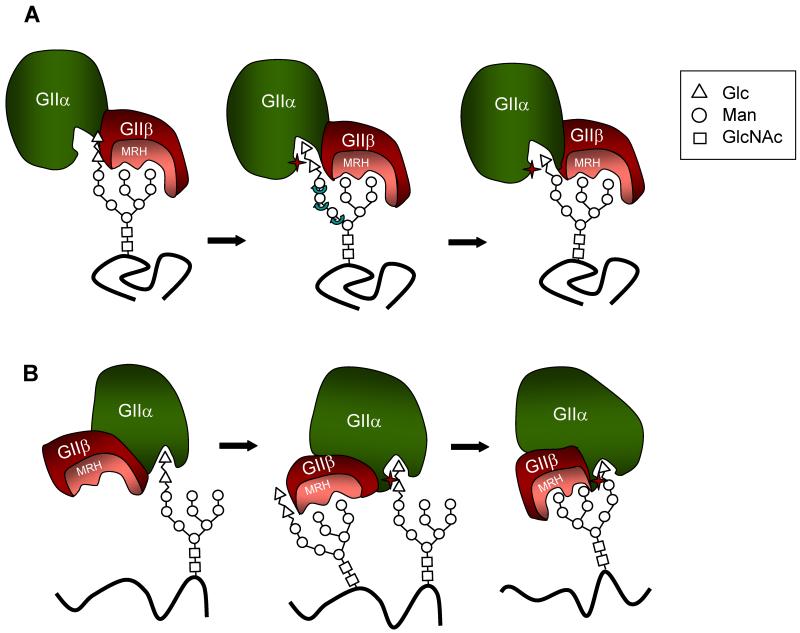
Fig. 3.
Models proposed for GIIβ-mediated enhancement of N-glycan deglucosylation. (A) Upon binding mannose units in the B and/or C arms of the glycan, the GIIβ MRH domain presents bonds to be cleaved to the GIIα catalytic site (star). Please note that in this model a rotation of A arm is required for both cleavages to proceed. (B) Binding of a glycan to the GIIβ MRH domain would result in a conformational change in their GIIβ subunit, which in turn would modify GIIα structure thus activating the catalytic site and allowing hydrolysis of the middle Glc residue in another glycan. Cleavage of the inner Glc in this last glycan would be enhanced by the interaction of Man units in its own B and/or C arms with the MRH domain as the bond to be cleaved and the arms lie on the same face.