Figure 1. Characteristics of ERdj proteins.
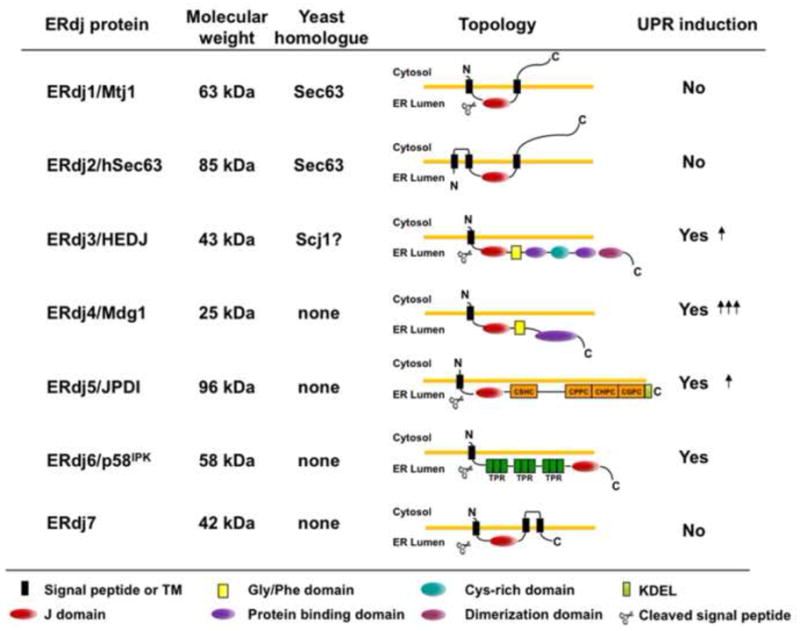
Seven DnaJ-like proteins have been identified in the mammalian ER. DnaJ proteins can be divided into three subgroups based on the similarities with E. coli DnaJ, the founding member of this family [77]. Type I proteins contain all three domains: an N-terminal J domain, a glycine/phenylalanine rich domain (yellow square), and a substrate binding domain, which is intersected with a cysteine-rich Zn2+ binding domain (light blue circle); type II proteins contain the first two domains and often bind to unfolded substrates, although this region is more poorly defined; and type III proteins have only a J domain, which can occur anywhere in the protein. In the mammalian ER most of the ERdjs are type III except for ERdj3 (type I) and ERdj4 (type II). ERdj5 contains four thioredoxin domains (orange squares) and ERdj6 has three tetratricopeptide domains (dark green squares). The detailed mechanism of their function can be found in the text.
