Figure 3.
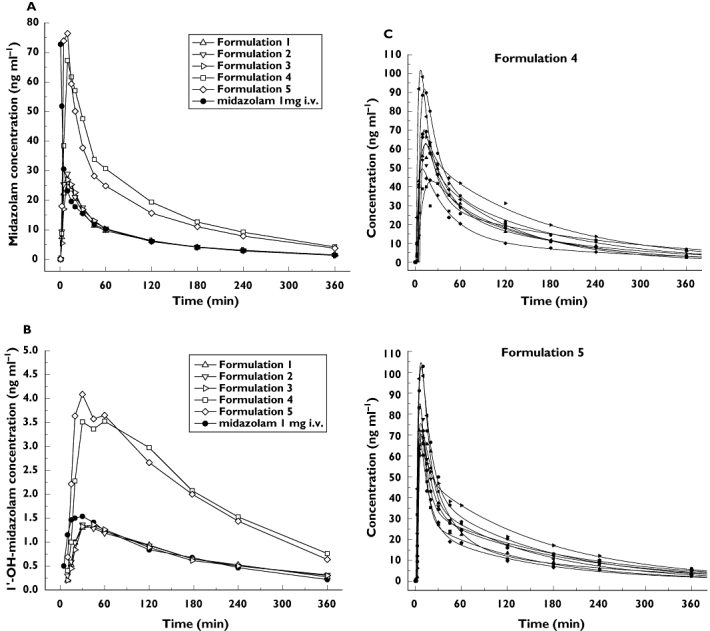
Kinetics of MDZ (A) and 1′-OH-MDZ (B) after intravenous administration of 1 mg MDZ and intranasal administration of 1 mg MDZ (formulations 1–3) and 3 mg MDZ (formulations 4 and 5). Fitting curves (C) generated by a two-compartmental pharmacokinetic model applied to the individual concentration–time data of the 3 mg nasal formulation without (formulation 4) and with chitosan (formulation 5). The absorption enhancer chitosan decreases variability in the early absorption phase, and significantly reduces tmax and increases Cmax
