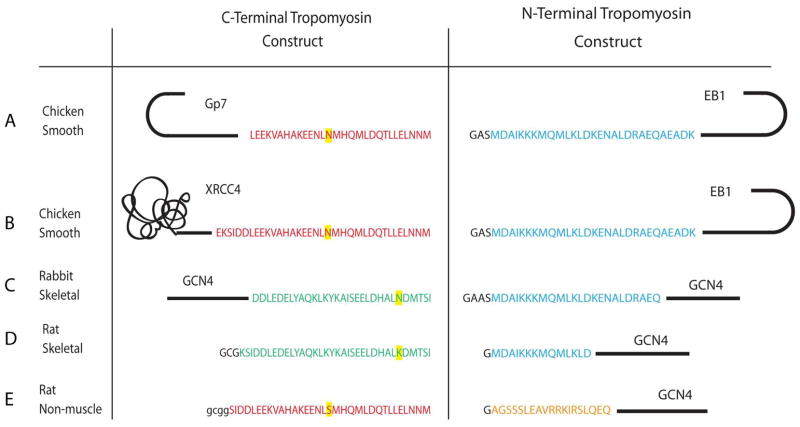
Figure 1.
Present and earlier constructs used in the structural studies of the tropomyosin overlap region. A) GP7 and EB1 fusions of avian smooth muscle tropomyosin. B) XRCC4 and EB1 fusions of avian smooth muscle tropomyosin. C) GCN4 fusions of rabbit skeletal muscle tropomyosin utilized in the X-ray structural determination (29). D) Rat skeletal muscle tropomyosin constructs used in the NMR structural determination (27). E) Rat non-muscle tropomyosin constructs used in the NMR structural study (28). These different tropomyosin isoforms are the consequence of different exon usage. The N-terminal sequence is identical in both the skeletal and smooth muscle isoforms (blue) and is encoded by exons 1a. The N-terminal sequence of the non-muscle isoform is encoded by exons 1b (orange). The C-terminal sequence of skeletal (green) and smooth muscle tropomyosin (red) are encoded by exons 9a and 9d respectively. Rat non-muscle tropomyosin uses the same C-terminal exon as chicken smooth muscle tropomyosin (10). Different amino acids found in the same exon from different species are highlighted in yellow.