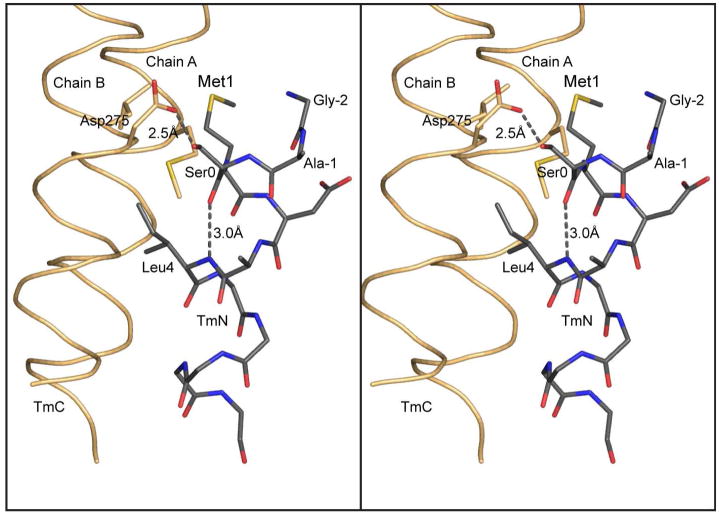
Figure 6.
Hydrogen bonding pattern at the N-terminus of tropomyosin. This figure shows a stereo close-up of the hydrogen bonding pattern associated with the N-terminal helix of tropomyosin and the Gly-Ala-Ser addition that is required to allow bacterially expressed tropomyosin to assemble into filaments. As seen here, the additional serine residue maintains the N-terminus of tropomyosin in an α-helical conformation. This is accomplished through an intrahelical hydrogen bond between the carbonyl oxygen of Ser0 and the amide hydrogen of Leu4, together with a hydrogen bond between Oδ1 of Asp275 and Oγ Ser0. In this conformation, Met1 lies in a hydrophobic pocket which will contribute to the hydrophobic stabilization of the complex. In nature, acetylation of the N-terminus would provide a terminal carbonyl oxygen that would be able to hydrogen bond back to the backbone amide hydrogen four residues away and thus allow Met 1 to remain in an α-helical conformation.