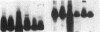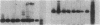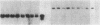Abstract
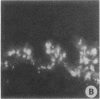
Clara cell 10-kD protein (cc10kD), a secretory phospholipase A2 inhibitor, is suggested to be the human counterpart of rabbit uteroglobin (UG). Because cc10kD is expressed constitutively at a very high level in the human respiratory epithelium, the 5' region of its gene may be useful in achieving organ-specific expression of recombinant DNA in gene therapy of diseases such as cystic fibrosis. However, it is important to establish the tissue-specific expression of this gene before designing gene transfer experiments. Since the UG gene in the rabbit is expressed in many other organs besides the lung and the endometrium, we investigated the organ and tissue specificity of human cc10kD gene expression using polymerase chain reaction, nucleotide sequence analysis, immunofluorescence, and Northern blotting. Our results indicate that, in addition to the lung, cc10kD is expressed in several nonrespiratory organs, with a distribution pattern very similar, if not identical, to that of UG in the rabbit. These results underscore the necessity for more detailed analyses of the 5' region of the human cc10kD gene before its usefulness in gene therapy could be fully assessed. These data also suggest that cc10kD and UG may have similar physiological function(s).
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly A., Atger M., Atger P., Cerbon M. A., Alizon M., Vu Haï M. T., Logeat F., Milgrom E. The rabbit uteroglobin gene. Structure and interaction with the progesterone receptor. J Biol Chem. 1983 Sep 10;258(17):10384–10389. [PubMed] [Google Scholar]
- Beier H. M., Bohn H., Müller W. Uteroglobin-like antigen in the male genital tract secretions. Cell Tissue Res. 1975 Dec 29;165(1):1–11. doi: 10.1007/BF00222795. [DOI] [PubMed] [Google Scholar]
- Beier H. M. Uteroglobin: a hormone-sensitive endometrial protein involved in blastocyst development. Biochim Biophys Acta. 1968 Jun 26;160(2):289–291. doi: 10.1016/0005-2795(68)90108-6. [DOI] [PubMed] [Google Scholar]
- Bloch W. A biochemical perspective of the polymerase chain reaction. Biochemistry. 1991 Mar 19;30(11):2735–2747. doi: 10.1021/bi00225a001. [DOI] [PubMed] [Google Scholar]
- Cabré F., Moreno J. J., Carabaza A., Ortega E., Mauleón D., Carganico G. Antiflammins. Anti-inflammatory activity and effect on human phospholipase A2. Biochem Pharmacol. 1992 Aug 4;44(3):519–525. doi: 10.1016/0006-2952(92)90444-n. [DOI] [PubMed] [Google Scholar]
- Camussi G., Tetta C., Baglioni C. Antiflammins inhibit synthesis of platelet-activating factor and intradermal inflammatory reactions. Adv Exp Med Biol. 1990;279:161–172. doi: 10.1007/978-1-4613-0651-1_10. [DOI] [PubMed] [Google Scholar]
- Camussi G., Tetta C., Bussolino F., Baglioni C. Antiinflammatory peptides (antiflammins) inhibit synthesis of platelet-activating factor, neutrophil aggregation and chemotaxis, and intradermal inflammatory reactions. J Exp Med. 1990 Mar 1;171(3):913–927. doi: 10.1084/jem.171.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. C., Ni M., Miele L., Cordella-Miele E., Ferrick M., Mukherjee A. B., Nussenblatt R. B. Effects of antiflammins on endotoxin-induced uveitis in rats. Arch Ophthalmol. 1991 Feb;109(2):278–281. doi: 10.1001/archopht.1991.01080020124058. [DOI] [PubMed] [Google Scholar]
- Daniel J. C., Jr, Milazzo J. T. Continuity of a rabbit antigen between generations. Cancer Res. 1976 Sep;36(9 Pt 2):3409–3411. [PubMed] [Google Scholar]
- DeMayo F. J., Damak S., Hansen T. N., Bullock D. W. Expression and regulation of the rabbit uteroglobin gene in transgenic mice. Mol Endocrinol. 1991 Mar;5(3):311–318. doi: 10.1210/mend-5-3-311. [DOI] [PubMed] [Google Scholar]
- Dhanireddy R., Kikukawa T., Mukherjee A. B. Detection of a rabbit uteroglobin-like protein in human neonatal tracheobronchial washings. Biochem Biophys Res Commun. 1988 May 16;152(3):1447–1454. doi: 10.1016/s0006-291x(88)80448-0. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Ialenti A. Selective inhibition of inflammatory reactions by vasocortin and antiflammin 2. Prog Clin Biol Res. 1990;349:81–90. [PubMed] [Google Scholar]
- Dveksler G. S., Basile A. A., Dieffenbach C. W. Analysis of gene expression: use of oligonucleotide primers for glyceraldehyde-3-phosphate dehydrogenase. PCR Methods Appl. 1992 May;1(4):283–285. doi: 10.1101/gr.1.4.283. [DOI] [PubMed] [Google Scholar]
- Guy J., Dhanireddy R., Mukherjee A. B. Surfactant-producing rabbit pulmonary alveolar type II cells synthesize and secrete an antiinflammatory protein, uteroglobin. Biochem Biophys Res Commun. 1992 Dec 15;189(2):662–669. doi: 10.1016/0006-291x(92)92252-s. [DOI] [PubMed] [Google Scholar]
- Hagen G., Wolf M., Katyal S. L., Singh G., Beato M., Suske G. Tissue-specific expression, hormonal regulation and 5'-flanking gene region of the rat Clara cell 10 kDa protein: comparison to rabbit uteroglobin. Nucleic Acids Res. 1990 May 25;18(10):2939–2946. doi: 10.1093/nar/18.10.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ialenti A., Doyle P. M., Hardy G. N., Simpkin D. S., Di Rosa M. Anti-inflammatory effects of vasocortin and nonapeptide fragments of uteroglobin and lipocortin I (antiflammins). Agents Actions. 1990 Jan;29(1-2):48–49. doi: 10.1007/BF01964716. [DOI] [PubMed] [Google Scholar]
- Kay E., Feigelson M. An estrogen modulated protein in rabbit oviducal fluid. Biochim Biophys Acta. 1972 Jul 21;271(2):436–441. doi: 10.1016/0005-2795(72)90219-x. [DOI] [PubMed] [Google Scholar]
- Kikukawa T., Cowan B. D., Tejada R. I., Mukherjee A. B. Partial characterization of a uteroglobin-like protein in the human uterus and its temporal relationship to prostaglandin levels in this organ. J Clin Endocrinol Metab. 1988 Aug;67(2):315–321. doi: 10.1210/jcem-67-2-315. [DOI] [PubMed] [Google Scholar]
- Kirchner C., Schroer H. G. Uterine secretion-like proteins in the seminal plasma of the rabbit. J Reprod Fertil. 1976 Jul;47(2):325–330. doi: 10.1530/jrf.0.0470325. [DOI] [PubMed] [Google Scholar]
- Kirchner C. Uteroglobin in the rabbit. II. Intracellular localization in the uterus after hormone treatment. Cell Tissue Res. 1976 Jul 30;170(3):425–434. doi: 10.1007/BF00219421. [DOI] [PubMed] [Google Scholar]
- Krishnan R. S., Daniel J. C., Jr "Blastokinin": inducer and regulator of blastocyst development in the rabbit uterus. Science. 1967 Oct 27;158(3800):490–492. doi: 10.1126/science.158.3800.490. [DOI] [PubMed] [Google Scholar]
- Levin S. W., Butler J. D., Schumacher U. K., Wightman P. D., Mukherjee A. B. Uteroglobin inhibits phospholipase A2 activity. Life Sci. 1986 May 19;38(20):1813–1819. doi: 10.1016/0024-3205(86)90135-9. [DOI] [PubMed] [Google Scholar]
- Lloret S., Moreno J. J. In vitro and in vivo effects of the anti-inflammatory peptides, antiflammins. Biochem Pharmacol. 1992 Oct 6;44(7):1437–1441. doi: 10.1016/0006-2952(92)90546-u. [DOI] [PubMed] [Google Scholar]
- Manjunath R., Levin S. W., Kumaroo K. K., Butler J. D., Donlon J. A., Horne M., Fujita R., Schumacher U. K., Mukherjee A. B. Inhibition of thrombin-induced platelet aggregation by uteroglobin. Biochem Pharmacol. 1987 Mar 1;36(5):741–746. doi: 10.1016/0006-2952(87)90728-3. [DOI] [PubMed] [Google Scholar]
- Manyak M. J., Kikukawa T., Mukherjee A. B. Expression of a uteroglobin-like protein in human prostate. J Urol. 1988 Jul;140(1):176–182. doi: 10.1016/s0022-5347(17)41522-9. [DOI] [PubMed] [Google Scholar]
- Miele L., Cordella-Miele E., Facchiano A., Mukherjee A. B. Inhibition of phospholipase A2 by uteroglobin and antiflammin peptides. Adv Exp Med Biol. 1990;279:137–160. doi: 10.1007/978-1-4613-0651-1_9. [DOI] [PubMed] [Google Scholar]
- Miele L., Cordella-Miele E., Facchiano A., Mukherjee A. B. Novel anti-inflammatory peptides from the region of highest similarity between uteroglobin and lipocortin I. Nature. 1988 Oct 20;335(6192):726–730. doi: 10.1038/335726a0. [DOI] [PubMed] [Google Scholar]
- Miele L., Cordella-Miele E., Mukherjee A. B. Uteroglobin: structure, molecular biology, and new perspectives on its function as a phospholipase A2 inhibitor. Endocr Rev. 1987 Nov;8(4):474–490. doi: 10.1210/edrv-8-4-474. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. B., Cordella-Miele E., Kikukawa T., Miele L. Modulation of cellular response to antigens by uteroglobin and transglutaminase. Adv Exp Med Biol. 1988;231:135–152. doi: 10.1007/978-1-4684-9042-8_11. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. B., Laki K., Agrawal A. K. Possible mechanism of success of an allotransplantation in nature: mammalian pregnancy. Med Hypotheses. 1980 Oct;6(10):1043–1055. doi: 10.1016/0306-9877(80)90017-1. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. B., Ulane R. E., Agrawal A. K. Role of uteroglobin and transglutaminase in masking the antigenicity of implanting rabbit embryos. Am J Reprod Immunol. 1982 Jun;2(3):135–141. doi: 10.1111/j.1600-0897.1982.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee D. C., Agrawal A. K., Manjunath R., Mukherjee A. B. Suppression of epididymal sperm antigenicity in the rabbit by uteroglobin and transglutaminase in vitro. Science. 1983 Feb 25;219(4587):989–991. doi: 10.1126/science.6130601. [DOI] [PubMed] [Google Scholar]
- Nordlund-Möller L., Andersson O., Ahlgren R., Schilling J., Gillner M., Gustafsson J. A., Lund J. Cloning, structure, and expression of a rat binding protein for polychlorinated biphenyls. Homology to the hormonally regulated progesterone-binding protein uteroglobin. J Biol Chem. 1990 Jul 25;265(21):12690–12693. [PubMed] [Google Scholar]
- Noske I. G., Feigelson M. Immunological evidence of uteroglobin (blastokinin) in the male reproductive tract and in nonreproductive ductal tissues and their secretions. Biol Reprod. 1976 Dec;15(5):704–713. doi: 10.1095/biolreprod15.5.704. [DOI] [PubMed] [Google Scholar]
- Noske I. G., Gooding M. Evidence of a uteroglobin-like protein in epithelial cells of reproductive and non-reproductive tissues of the rabbit. J Reprod Fertil. 1978 Sep;54(1):193–196. doi: 10.1530/jrf.0.0540193. [DOI] [PubMed] [Google Scholar]
- Perretti M., Becherucci C., Mugridge K. G., Solito E., Silvestri S., Parente L. A novel anti-inflammatory peptide from human lipocortin 5. Br J Pharmacol. 1991 Jun;103(2):1327–1332. doi: 10.1111/j.1476-5381.1991.tb09788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann E., Geetha V., Pencev D., Warabi H., Mato J., Hirata F., Brownstein M., Manjunath R., Mukherjee A., Liotta L. Adherence and regulation of leukotaxis. Agents Actions Suppl. 1983;12:106–120. doi: 10.1007/978-3-0348-9352-7_6. [DOI] [PubMed] [Google Scholar]
- Seilhamer J. J., Randall T. L., Yamanaka M., Johnson L. K. Pancreatic phospholipase A2: isolation of the human gene and cDNAs from porcine pancreas and human lung. DNA. 1986 Dec;5(6):519–527. doi: 10.1089/dna.1.1986.5.519. [DOI] [PubMed] [Google Scholar]
- Singh G., Katyal S. L., Brown W. E., Kennedy A. L., Singh U., Wong-Chong M. L. Clara cell 10 kDa protein (CC10): comparison of structure and function to uteroglobin. Biochim Biophys Acta. 1990 Jul 6;1039(3):348–355. doi: 10.1016/0167-4838(90)90270-p. [DOI] [PubMed] [Google Scholar]
- Singh G., Katyal S. L., Brown W. E., Phillips S., Kennedy A. L., Anthony J., Squeglia N. Amino-acid and cDNA nucleotide sequences of human Clara cell 10 kDa protein. Biochim Biophys Acta. 1988 Sep 7;950(3):329–337. doi: 10.1016/0167-4781(88)90129-7. [DOI] [PubMed] [Google Scholar]
- Singh G., Singh J., Katyal S. L., Brown W. E., Kramps J. A., Paradis I. L., Dauber J. H., Macpherson T. A., Squeglia N. Identification, cellular localization, isolation, and characterization of human Clara cell-specific 10 KD protein. J Histochem Cytochem. 1988 Jan;36(1):73–80. doi: 10.1177/36.1.3275712. [DOI] [PubMed] [Google Scholar]
- Stripp B. R., Sawaya P. L., Luse D. S., Wikenheiser K. A., Wert S. E., Huffman J. A., Lattier D. L., Singh G., Katyal S. L., Whitsett J. A. cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem. 1992 Jul 25;267(21):14703–14712. [PubMed] [Google Scholar]
- Suske G., Wenz M., Cato A. C., Beato M. The uteroglobin gene region: hormonal regulation, repetitive elements and complete nucleotide sequence of the gene. Nucleic Acids Res. 1983 Apr 25;11(8):2257–2271. doi: 10.1093/nar/11.8.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetta C., Camussi G., Bussolino F., Herrick-Davis K., Baglioni C. Inhibition of the synthesis of platelet-activating factor by anti-inflammatory peptides (antiflammins) without methionine. J Pharmacol Exp Ther. 1991 May;257(2):616–620. [PubMed] [Google Scholar]
- Umland T. C., Swaminathan S., Furey W., Singh G., Pletcher J., Sax M. Refined structure of rat Clara cell 17 kDa protein at 3.0 A resolution. J Mol Biol. 1992 Mar 20;224(2):441–448. doi: 10.1016/0022-2836(92)91006-b. [DOI] [PubMed] [Google Scholar]
- Vasanthakumar G., Manjunath R., Mukherjee A. B., Warabi H., Schiffmann E. Inhibition of phagocyte chemotaxis by uteroglobin, an inhibitor of blastocyst rejection. Biochem Pharmacol. 1988 Feb 1;37(3):389–394. doi: 10.1016/0006-2952(88)90204-3. [DOI] [PubMed] [Google Scholar]
- Wolf M., Klug J., Hackenberg R., Gessler M., Grzeschik K. H., Beato M., Suske G. Human CC10, the homologue of rabbit uteroglobin: genomic cloning, chromosomal localization and expression in endometrial cell lines. Hum Mol Genet. 1992 Sep;1(6):371–378. doi: 10.1093/hmg/1.6.371. [DOI] [PubMed] [Google Scholar]