Fig. 1.
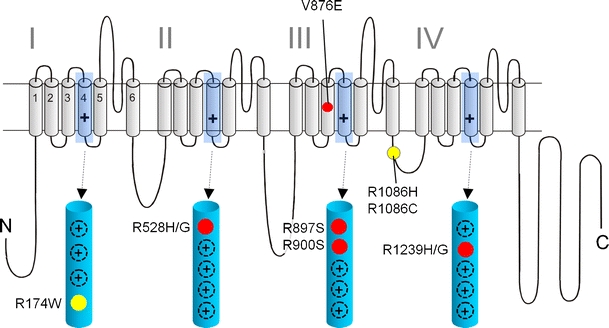
Mutations in Ca2+ channel Cav1.1 α1 subunits identified in patients with HPP-1 and MHS: a folding model of α1-subunits based on hydrophobicity analysis is shown. Plus sign indicates several positive charges in the transmembrane S4 helices within the hydrophobic repeats I–IV. S4 helices and their positively charged residues are shown in the enlarged structures. Together with S1, S2, and S3 helices, they form the four voltage-sensing domains of the channel controlling the opening and closing of a single pore domain formed by S5 and S6 helices together with the connecting linkers. HPP-1 mutations are indicated in red; MHS mutations are shown in yellow. The location of other positive charges in the S4 domains is indicated as black circles (plus sign)
