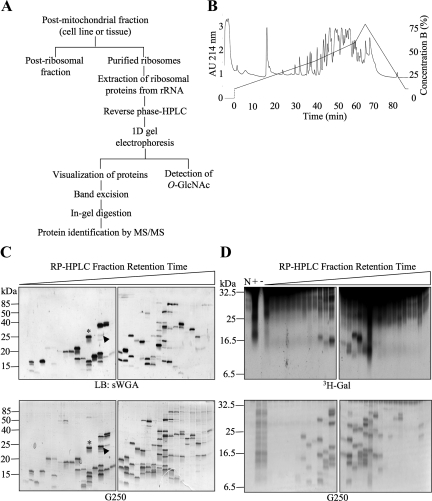
Figure 2.
Extensive separation of ribosomes allows the detection of multiple individual O-GlcNAcylated proteins. (A) Strategy for separation and identification of ribosomal proteins modified with O-GlcNAc. (B) Representative chromatogram obtained after HPLC separation of a purified rat liver ribosomal fraction over a reverse-phase C8 column. (C) Reverse-phase HPLC fractions containing ribosomal protein peaks from HeLa cells were separated by one-dimensional gel electrophoresis and subjected to lectin blot (LB) with sWGA (top panels). Fractions were loaded in subsequent order of retention time (early to late from left to right). Comparison with the corresponding G250-stained gel (bottom panels) shows that many, but not all, proteins present in the fractions contain O-GlcNAc. Two examples (asterisk and arrowhead) show that the lectin signal for O-GlcNAc has different stoichiometries when compared with equivalent levels of total protein. (D) Fractions as in C were subjected to galactosyltransferase labeling in the presence of UDP-[3H]Gal (top panels). Bottom panels show G250 protein stain of the top panel. No substrate (N), total preparation (previous to RP-HPLC) with galactosyltransferase (+), and total preparation without galactosyltransferase (−) controls are included. Data shown are representative of at least three independent experiments.