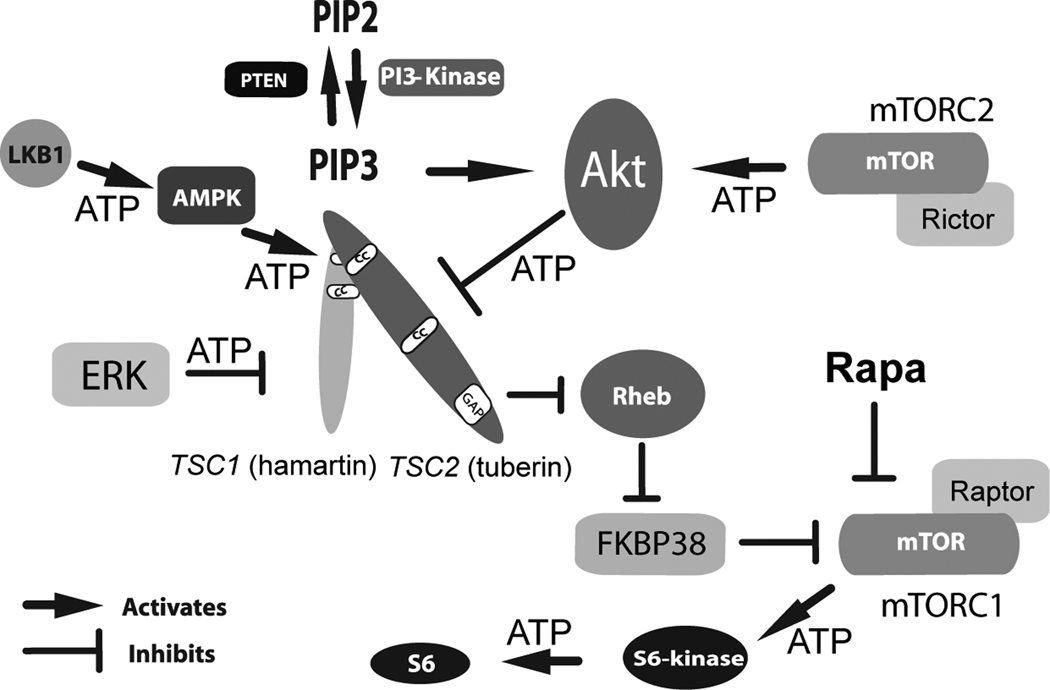
Figure 1. Schematic of Upstream and Downstream Signaling Pathways in TSC.
A complex series of phosphorylation events and other protein interactions regulate hamartin/tuberin and activity of the mTOR kinase. Initial growth factor binding to transmembrane receptors (not shown) activates PI-3 Kinase resulting in increased production of PIP3 with AKT activation that directly phosphorylates and inhibits tuberin. ERK can also phosphorylate and inactivate tuberin. In contrast, AMPK phosphorylation at distinct amino acid residues serves to activate tuberin. Loss of the TSC1 or TSC2 genes leads to constitutive activation of mTOR within mTORC1 with greatly increased levels of phosphorylated ribosomal S6-kinase and phosphorylated ribosomal S6. Rapamycin inhibits mTOR activity within mTORC1 to restore inhibition of this kinase and downstream components within this signaling pathway. ATP indicates phosphorylation events.
AKT (proto-oncogene also known as PKB); AMPK, AMP-activated protein kinase; ERK, Extracellular Signal-Regulated Kinases; FKBP38, FK506-binding protein 38; LKB1, Peutz-Jeghers Syndrome Kinase; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; mTORC2, mammalian target of rapamycin two; PIP2, phosphatidylinositol bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PTEN, phosphatase and tensin homolog; Rapa, rapamycin; Raptor, regulatory associated protein of mTOR, Rictor, rapamycin-insensitive companion of mTOR; Rheb, Ras homolog enriched in brain; TSC1, (hamartin); TUBEROUS SCLEROSIS COMPLEX GENE ONE; TSC2, (tuberin) TUBEROUS SCLEROSIS COMPLEX GENE TWO.