Abstract
Background: Maternal folate nutritional status and prenatal lead exposure can influence fetal development and subsequent health. The methylenetetrahydrofolate reductase (MTHFR) gene is important for folate metabolism, and 2 common polymorphisms, C677T and A1298C, reduce enzymatic activity; C677T is present at high penetrance in Mexican populations.
Objective: The objective of this study was to examine potential links between maternal and child MTHFR polymorphisms and child neurodevelopment in a lead-exposed population.
Design: Data regarding MTHFR polymorphisms C677T and A1298C, peri- and postnatal lead measures, and Bayley Mental Development Index at 24 mo of age (MDI-24) scores were available for 255 mother-child pairs who participated in the ELEMENT (Early Life Exposures in Mexico to Environmental Toxicants) study during 1994–1995.
Results: In covariate-adjusted regression models, maternal MTHFR 677 genotype predicted MDI-24 scores, in which each copy of the maternal MTHFR 677T variant allele was associated with lower MDI-24 scores (β = −3.52; 95% CI: −6.12, −0.93; P = 0.004). Maternal MTHFR haplotype also predicted MDI-24 scores (mean ± SE: 93.3 ± 1.2 for 677C-1298A compared with 89.9 ± 0.8 for 677T-1298A; P < 0.05). MDI-24 scores were not associated with maternal MTHFR 1298 genotype or child MTHFR genotypes. We did not observe significant MTHFR genotype × lead interactions with respect to any of the subject biomarkers of lead exposure.
Conclusions: The maternal MTHFR 677T allele is an independent predictor of poorer child neurodevelopment at 24 mo. These results suggest that maternal genetic variations in folate metabolism during pregnancy may program offspring neurodevelopment trajectories. Further research is warranted to determine the generalizability of these results across other populations.
INTRODUCTION
Nutritional status and environmental factors play a critical role in neurodevelopment (1, 2). The mammalian central nervous system is the most susceptible biological system to developmental injury from exogenous insults because of its complexity and reliance on numerous timed processes, such as neuronal differentiation, cell migration, and myelin deposition (3). Developmental lead exposure in animal models target numerous processes for cognitive neurodevelopment, such as long-term potentiation (4), neurogenesis (5), and expression profiles of glutamate receptor subunits (6). In addition, several epidemiologic studies have shown that maternal body burdens of lead during pregnancy can impair the neurobehavioral development of children and manifest as cognitive delays and a lower intelligence quotient (IQ) later in life (7–9). Of particular interest for neurodevelopment is the interplay between environmental toxicants, such as developmental lead exposure, and critical nutrients.
Adequate maternal folate nutritional status is a central contributor to fetal development and subsequent offspring health, especially concerning its role in reducing the incidence of neural tube defects (10). To meet the high daily requirement of folate for one-carbon reactions during fetal development, this micronutrient is actively transported across the placenta against a concentration gradient such that folate concentrations are typically 3 times higher in the umbilical cord than in the maternal circulation (11). Consequently, maternal folate status and/or polymorphisms in folate-metabolizing genes may affect nutrient delivery to the fetus and subsequent neurodevelopment.
The methylenetetrahydrofolate reductase (MTHFR) gene is critical to folate metabolism and contains 2 common missense polymorphisms, C677T and A1298C, each of which renders the enzyme functionally impaired (12–14). MTHFR is located at the major branch point of one-carbon metabolism, where folate in the 5,10,-methlyenetetrahydrofolate (5,10-methyleneTHF) isoforms can be used for nucleotide synthesis or, alternatively, can be reduced to 5-methylTHF and thereby commit folate solely to biomethylation reactions. Individuals who carry the MTHFR 677 TT variant genotype have reduced plasma and red blood cell folate concentrations, elevated plasma homocysteine concentrations (in individuals with low blood folate concentrations) (15–17), and differential coenzymatic forms of folate in red blood cells (18). In Mexican populations, the prevalence of the MTHFR C677T variant is reported to be one of the highest worldwide (19).
There are relatively few studies on the influence of maternal folate status and/or the MTHFR genotype on child neurocognitive development, especially with regards to the adverse effects of environmental lead exposure. In the central Philippines, folate deficiency was reported to increase the susceptibility to lead-associated cognitive declines in children (20). To gain a better understanding of other potential neurodevelopmental risk factors, the current study examined the influence of maternal and child MTHFR genotypes on child neurodevelopment at 24 mo of age. In addition, we examined potential interactions between the MTHFR genotype and prenatal lead concentrations that concern child neurodevelopment.
SUBJECTS AND METHODS
Sample population
The ELEMENT (Early Life Exposures in Mexico to Environmental Toxicants) study is a group of sequentially enrolled epidemiologic birth-cohort studies with the aim of investigating the influence of cumulative maternal lead burden on fetal and infant development. For the current study, which used data and biological samples from the first birth cohort, maternal/child pairs were recruited between 1994 and 1995 from 3 hospitals in Mexico City (Mexican Social Security Institute, Manuel Gea Gonzalez Hospital, and National Institute of Perinatology) which serve low-to-moderate-income populations. Exclusion criteria were as follows: factors that could interfere with maternal calcium metabolism; medical conditions that could cause low birth weight (<2000 g); logistic reasons that would interfere with data collection (households located outside the metropolitan area); delivery of a premature neonate (<37 wk) or an infant with an Apgar score at 5 min of ≤6; conditions that required placement in a neonatal intensive care unit; a physician's diagnosis of multiple fetuses; the intention not to breastfeed; preeclampsia; psychiatric, kidney, or cardiac diseases; gestational diabetes; a history of repeated urinary infections; a family or personal history of kidney-stone formation; a seizure disorder requiring daily medication; the ingestion of corticosteroids; or >140 mm Hg systolic or >90 mm Hg diastolic blood pressure. Of the initial 1382 mothers who remained eligible, 617 agreed to participate and continued in the birth-cohort study. Of these, DNA was extracted from 412 umbilical cord samples. Our data set consisted of 256 mother-child pairs who had complete MTHFR 677 and MTHFR 1298 genotype data and Bayley Mental Development Index at 24 mo of age (MDI-24) scores.
Blood lead measurements
Umbilical cord venous blood samples were collected in trace metal-free tubes at delivery. Blood samples were analyzed with an atomic absorption spectrometry instrument (Perkin-Elmer 3000; Perkin-Elmer, Chelmsford, MA) at the metals laboratory of the American British Cowdray Medical Center (Mexico City, Mexico). External blinded quality-control samples were provided throughout the study period by the Maternal and Child Health Bureau (Rockville, MD) and the Wisconsin State Laboratory of Hygiene Cooperative Blood Lead Proficiency Testing Program (Madison, WI).
Bone lead measurements
Maternal bone lead was measured noninvasively around 1 mo postpartum with a spot-source 109Cd K-X-ray fluorescence instrument constructed at Harvard University and installed in a research facility in the American British Cowdray Medical Center. The physical principles, technical specifications, and validation of this and other similar K-X-ray fluorescence instruments have been described in detail elsewhere (21). For this study, 30-min measurements were taken at the midshaft of the left tibia (cortical bone) and the left patella (trabecular bone). Analysis of means and standard deviations of phantom-calibrated measurements did not disclose any significant shift in accuracy or precision. As a quality-control measure, any tibia lead measurements with an uncertainty >10 μg/g or any patella lead measurements with an uncertainty >15 μg/g were excluded as a routine practice (22). Only 3% of tibia lead concentrations and 6% of patella lead concentrations were excluded because of measurement uncertainty.
Measurements of childhood development and potential confounders
The Bayley Scales of Infant Development (BSID-II) is a revision and restandardization of the BSID, the most widely used test of infant development, and our protocol for its implementation has been described previously by our group (7). The revised scale can be used to assess the development of children between the ages of 1 and 42 mo. Scores have been shown to be sensitive to a variety of prenatal, perinatal, and postnatal insults, including lead exposure (8, 23, 24). The BSID-II has also been used in numerous cross-cultural studies of lead and child development (25, 26). A Spanish version of the BSID-II was developed and validated by our research group before this study. MDI-24 scores were used as the primary child-development endpoint in this study. An interviewer-administered survey collected at 1 mo postpartum provided information on sociodemographic characteristics, reproductive history, and other factors that may constitute potential cofounders of the relation between lead and child development. Maternal IQ was assessed by using the Information, Comprehension, Similarities, and Block Design components of the Wechsler Adult Intelligence Score, which has been translated into Spanish and used in Mexico (7).
DNA extraction and genotyping
DNA extraction was performed in the Harvard-Partners Center for Genetics and Genomics. High-molecular-weight DNA was extracted with commercially available PureGene Kits (Gentra Systems, Minneapolis, MN) from the white blood cells of archived umbilical cord blood samples that were collected at delivery. A TaqMan platform (Applied Biosystems, Carlsbad, CA) was used to genotype the MTHFR single-nucleotide polymorphisms C677T (rs1801133) and A1298C (rs1801131) as described elsewhere (27). The combinations of wild-type genotypes are abbreviated as follows: for example, 677 CC and 1298 AA is abbreviated CCAA, and 677 CT and 1298 AC is abbreviated CTAC. Allele distribution was previously shown to be in Hardy-Weinberg equilibrium for this study population (27).
Dietary assessment
Food-frequency questionnaire
Maternal dietary folate intake was estimated by using a semiquantitative food-frequency questionnaire (FFQ) administered at 1 mo postpartum by trained interviewers. The FFQ was developed on the basis of a previously validated instrument (28, 29) and subsequently adapted and validated for use in Spanish-speaking, adult women (30, 31). The FFQ queried usual frequency over the previous year of consuming 116 foods and beverages that are typical in Mexican diets with 10 response options that ranged from never to monthly, weekly, and daily intakes. Food folate intakes were then calculated on the basis of a nutrient database that comprised reference data from the US Department of Agriculture and the National Institute of Nutrition in Mexico (30).
Folate bioequivalence
The bioavailability of folic acid derived from dietary supplements is much higher than from food folate (32, 33). To account for bioavailability differences, all folate data were converted to dietary folate equivalents (DFEs) as calculated by the following formula: DFE = micrograms of food folate + 1.7 × micrograms of folic acid. Low folate intake was classified as <520 DFEs, which is the estimated average requirement (EAR) for pregnant women (34).
Dietary supplement use
Information for dietary supplementation use was collected as part of the FFQ, which determined the brand, frequency, and duration of prenatal supplement use over the previous year. The average daily intake of folic acid was calculated by the number of days that supplement use was reported, the number of pills per day, and the serving size from the product label (35).
Ethics
The study protocol was approved by the Ethics Committee of the National Institute of Public Health of Mexico, the participating hospitals, the Brigham and Women's Hospital, the Harvard School of Public Health, and the University of Michigan. All participating mothers received a detailed explanation of the study intent, research procedures, and counseling on how to reduce environmental lead exposure.
Statistics
Descriptive statistics for characteristics of the study sample were calculated separately for participating or nonparticipating individuals. Excluded individuals were those who were missing either MTHFR genotype data or child MDI-24 scores. Differences by participation status were tested by using Wilcoxon's rank-sum or chi-square tests for quantitative and categorical variables, respectively. Linear regression models were used to describe the relation between MTHFR genotype and child MDI-24 scores. In adjusted models, covariates were included on the basis of biological plausibility or those previously shown to be associated with child MDI scores in a previous study of prenatal lead exposure and child cognition (7). The included covariates were as follows: maternal age, maternal IQ, marital status, parity, gestational age, inadequate folate intake, and umbilical cord lead concentrations. Regression diagnostics were performed on all models to evaluate multicollinearity and violations of assumptions of the linear regression model. To test for potential interactions between the MTHFR genotype and bone and blood lead measures, interaction terms (MTHFR 677 × lead and MTHFR 1298 × lead) were included in separate analyses that predicted MDI-24 scores. Interaction terms between the MTHFR genotype and low folate intake were also investigated. Data were analyzed with SAS 9.1 software (2002–2003; SAS Institute, Cary, NC).
PHASE version 2.1.1 software (University of Washington, Seattle, WA) was used to construct haplotype estimates and allele frequencies for MTHFR C677T and A1298C. This program requires an input file with information on genotypes organized by the individual. The set was run in 100 iterations with a burn-in interval of 100 and a thinning interval of 1. The PHASE program uses a type of Markov chain–Monte Carlo algorithm to find the appropriate sample of individuals given the genotypes. The repetition of this process over enough intervals conveys an approximate sample, constructs a Markov chain with a stationary distribution, and conveys possible haplotype reconstructions (36, 37). Linear regression models were used to examine the relation between MTHFR haplotypes (n = 510) and child MDI-24 scores.
RESULTS
The characteristics of study participants (n = 255) and subjects who were excluded (n = 374) because of missing MDI-24 scores and/or MTHFR genotype data are presented in Table 1. Subjects who were excluded from the analyses and subjects who were included in the analyses were similar in such variables as maternal age, body mass index, IQ, and nutritional intake, child birth measures, and lead biomarkers. The use of dietary supplements containing folic acid was reported by 17.3% (n = 44) of the participating mothers. In study participants, supplement users had a higher mean of total folate intake than did nonsupplement users (mean ± SD: 492.9 ± 241.5 compared with 372.3 ± 138.6 DFEs/d, respectively; P < 0.001). With the use of the folate EAR for pregnant women of 520 DFEs/d, 65.9% of supplement users and 87.1% of nonusers were classified as having low folate intake (P < 0.001).
TABLE 1.
Characteristics of subjects by participation status1
| Participating (n = 255) |
Nonparticipating (n = 374) |
|||
| Variable | Values | n | Values | n |
| Mothers | ||||
| Age (y) | 24.6 ± 5.12 | 255 | 24.5 ± 5.1 | 374 |
| BMI (kg/m2) | 23.6 ± 3.5 | 205 | 23.5 ± 3.8 | 291 |
| Education (y) | 9.4 ± 3.1 | 255 | 9.1 ± 3.2 | 374 |
| IQ | 84.3 ± 23.5 | 253 | 85.1 ± 24.3 | 229 |
| Parity | 1.9 ± 1.1 | 255 | 2.1 ± 1.3 | 372 |
| Total protein (g/d) | 79.9 ± 31.6 | 253 | 76.1 ± 29.6 | 370 |
| Folate intake (DFEs/d) | 393.3 ± 167.0 | 253 | 369.6 ± 161.1 | 370 |
| Supplement users (%) | 17.3 | 44 | 15.8 | 59 |
| Food intake (DFEs/d) | 389.6 ± 185.7 | 44 | 399.5 ± 153.6 | 59 |
| Total intake (DFEs/d) | 492.9 ± 241.5 | 44 | 495.1 ± 244.2 | 59 |
| Low folate intake (%)3 | 65.9 | 29 | 67.8 | 40 |
| Non–supplement users (%) | 82.0 | 209 | 81.2 | 311 |
| Food intake (DFEs/d) | 372.3 ± 138.6 | 209 | 345.8 ± 127.2 | 311 |
| Total intake (DFEs/d) | 372.3 ± 138.64 | 209 | 345.8 ± 127.2 | 311 |
| Low folate intake (%)3 | 87.15 | 182 | 91.0 | 283 |
| Vitamin B-12 (μg/d) | 6.6 ± 3.0 | 253 | 6.0 ± 2.7 | 370 |
| Tibia lead (μg/g) | 10.5 ± 10.4 | 248 | 9.7 ± 9.9 | 366 |
| Patella lead (μg/g) | 14.7 ± 13.7 | 239 | 14.9 ± 16.6 | 348 |
| Children | ||||
| Birth weight (g) | 3144.3 ± 432.3 | 254 | 3128.3 ± 411.4 | 372 |
| Birth length (cm) | 50.3 ± 2.3 | 251 | 50.4 ± 2.4 | 368 |
| Gestational age (wk) | 39.2 ± 1.5 | 254 | 39.1 ± 1.5 | 365 |
| MDI-24 scores | 91.6 ± 14.1 | 255 | 92.0 ± 13.9 | 84 |
| Male (%) | 45.3 | 115 | 45.3 | 168 |
| Umbilical cord blood lead (μg/dL) | 6.7 ± 3.6 | 221 | 6.6 ± 3.6 | 298 |
IQ, intelligence quotient; DFEs, dietary folate equivalents; MDI-24, Bayley Mental Development Index at 24 mo of age.
Mean ± SD (all such values).
<520 DFEs/d, which is the estimated average requirement for pregnant women.
Significantly different from supplement users: 4P < 0.0001 (t test), 5P < 0.0005 (chi-square test).
Genotype frequencies for the C677T and A1298C polymorphisms in children and mothers are shown in Tables 2 and 3. For the C677T polymorphism, ≈15% of subjects carried the wild-type CC genotype, whereas >38% of subjects carried the variant TT genotype. Conversely, the majority of subjects (>77%) were carriers of the wild-type 1298C AA genotype, whereas only 1% of subjects carried the variant CC genotype. The frequencies of the 9 genotype combinations resulting from the C677T and A1298C polymorphisms are also presented in Tables 2 and 3. No subjects were homozygous recessive for both MTHFR genotypes (TTCC) or homozygous recessive for one MTHFR genotype and heterozygous for the other (TTAC and CTCC). The most common genotypes of children and mothers were TTAA (39.2% and 38.4%, respectively) and CTAA (31.8% and 34.9%, respectively). The majority of children and mothers were carriers of at least one 677T variant allele (85.5% and 86.2%, respectively).
TABLE 2.
Child MTHFR genotype frequencies
| 1298 |
||||
| 677 | AA | AC | CC | Total |
| % (n) | % (n) | |||
| CC | 6.67 (17) | 7.1 (18) | 0.8 (2) | 14.5 (37) |
| CT | 31.8 (81) | 14.5 (37) | — | 46.3 (118) |
| TT | 39.2 (100) | — | — | 39.2 (100) |
| Total | 77.7 (198) | 21.6 (55) | 0.8 (2) | 255 |
TABLE 3.
Maternal MTHFR genotype frequencies
| 1298 |
||||
| 677 | AA | AC | CC | Total |
| % (n) | % (n) | |||
| CC | 8.2 (21) | 4.3 (11) | 1.2 (3) | 13.7 (35) |
| CT | 34.9 (89) | 12.9 (33) | — | 47.8 (122) |
| TT | 38.4 (98) | — | — | 38.4 (98) |
| Total | 81.6 (208) | 17.3 (44) | 1.2 (3) | 255 |
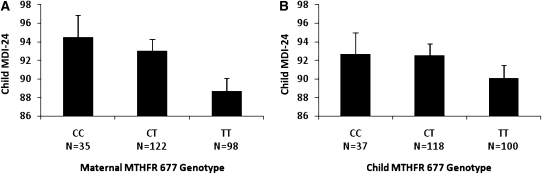
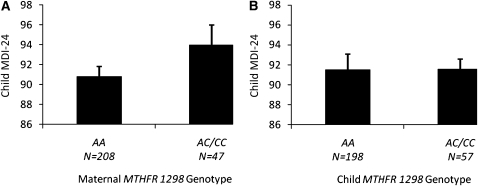
The unadjusted mean MDI-24 scores with maternal and child MTHFR 677 genotypes are shown in Figure 1. In mothers, we observed a MTHFR 677 genotype-dependent decrease in MDI-24 scores such that a higher number of copies of the 677T variant allele was associated with lower MDI-24 scores (P for trend = 0.01). Children born to mothers carrying the 677 TT genotype had mean (±SE) MDI-24 scores 5.8 points lower (88.7 ± 1.4) than the MDI-24 scores of children of mothers carrying the 677 CC genotype (94.5 ± 2.4; P = 0.04). For the maternal MTHFR 1298 genotype, unadjusted homozygous-dominant models (AA compared with AC/CC) revealed a modest but unsignificant difference between maternal genotypes and MDI-24 scores (mean ± SE: AA = 90.8 ± 1.0 compared with AC/CC = 94.7 ± 2.0, P = 0.09; Figure 2). We did not observe any significant associations between child MTHFR 677 or MTFHR 1298 genotypes and MDI-24 scores.
FIGURE 1.
A: Unadjusted mean scores of the Bayley Mental Development Index at 24 mo of age (MDI-24) by maternal MTHFR 677 genotype (P for trend = 0.01; TT compared with CC or CT; P < 0.05). B: Unadjusted mean MDI-24 scores by child MTHFR 677 genotype (P for trend = 0.22).
FIGURE 2.
A: Unadjusted mean scores of the Bayley Mental Development Index at 24 mo of age (MDI-24) by maternal MTHFR 1298 genotype (P = 0.09). B: Unadjusted mean MDI-24 scores by child MTHFR 1298 genotype (P = 0.98).
Multiple regression models of maternal and child MTHFR genotypes and MDI-24 scores adjusted for maternal age, IQ, and low folate intake, gestational age, and parity are shown in Table 4. We observed a −3.52 point reduction in MDI-24 scores for each additional copy of the maternal MTHFR 677T variant allele after controlling for covariates (P = 0.004). No significant associations were observed between other MTHFR genotypes and MDI-24 scores. Low maternal folate intake was not associated with MDI-24 scores. No significant interactions were shown between MTHFR genotype × low folate intake and MTHFR genotype × prenatal lead concentrations on MDI-24 scores.
TABLE 4.
Multiple regression models of maternal and child MTHFR genotypes and Bayley Mental Development Index at 24 mo of age scores1
| Maternal |
Child |
|||
| Variable | β (95% CI) | P | β (95% CI) | P |
| MTHFR 6772 | −3.52 (−6.12, −0.93) | 0.004 | −1.73 (−4.31, 0.85) | 0.19 |
| Maternal age | −0.23 (−0.62, 0.17) | 0.26 | −0.22 (−0.62, 0.18) | 0.28 |
| Gestational age | 0.72 (−0.54, 21.98) | 0.26 | 0.86 (−0.42, 2.13) | 0.19 |
| Umbilical cord lead | −0.71 (−1.20, −0.21) | 0.005 | −0.71 (−1.21, −0.21) | 0.006 |
| Maternal IQ | 0.12 (0.04, 0.20) | 0.002 | 0.13 (0.05, 0.21) | 0.002 |
| Parity | −2.75 (−6.58, 1.08) | 0.16 | −2.67 (−6.56, 1.23) | 0.18 |
| Low folate intake3 | 1.32 (−3.60, 6.23) | 0.53 | 0.79 (−4.17, 5.75) | 0.75 |
| Marital status | 4.00 (0.11, 7.88) | 0.04 | 4.27 (0.35, 8.20) | 0.03 |
| MTHFR 12984 | −2.78 (−7.61, 2.04) | 0.26 | −0.33 (−4.57, 3.90) | 0.88 |
| Maternal age | −0.25 (−0.65, 0.15) | 0.22 | −0.23 (−0.63, 0.17) | 0.25 |
| Gestational age | 0.86 (−0.41, 2.14) | 0.18 | 0.86 (−0.43, 2.14) | 0.19 |
| Umbilical cord lead | −0.70 (−1.20, −0.20) | 0.007 | −0.73 (−1.22, −0.23) | 0.004 |
| Maternal IQ | 0.12 (0.04, 0.20) | 0.002 | 0.13 (0.05, 0.21) | 0.002 |
| Parity | −2.84 (−6.72, 1.30) | 0.15 | −2.94 (−6.83, 0.96) | 0.14 |
| Low folate intake3 | 0.63 (−4.33, 5.59) | 0.80 | 0.68 (−4.30, 5.67) | 0.79 |
| Marital status | 4.31 (0.38, 8.23) | 0.03 | 4.46 (0.53, 8.39) | 0.03 |
IQ, intelligence quotient.
CC compared with CT compared with TT.
<520 dietary folate equivalents/d, which is the estimated average requirement for pregnant women.
AA compared with AC/CC.
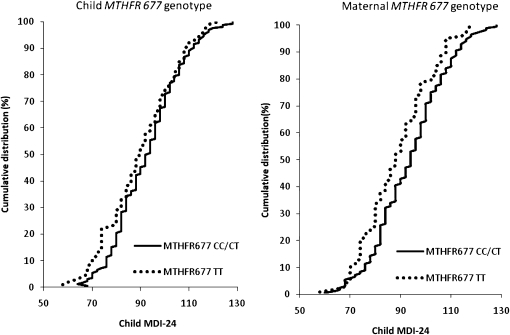
To further illustrate the influence of maternal MTHFR 677 genotypes on MDI-24 scores, we plotted the cumulative distributions of MDI-24 scores and compared the 677 CC/CT and TT genotypes in the 255 mother-child pairs (Figure 3). We observed a shift in the cumulative distribution of MDI-24 scores such that children born to mothers who were 677 TT carriers had poorer MDI-24 scores than did children born to mothers who were carriers of the 677 CC or CT genotypes (Figure 3B). The extent of shift in the cumulative distribution of MDI-24 scores was less apparent when the child MTHFR 677 genotype was plotted (Figure 3A).
FIGURE 3.
Cumulative frequency distributions of scores of the Bayley Mental Development Index at 24 mo of age (MDI-24) comparing MTHFR 677 CC/CT and TT genotypes in 256 mother-child pairs.
Finally, we examined the association of MTHFR haplotypes with child neurodevelopment (Table 5). The 677T-1298A haplotype was the most frequent allele predicted in our population for both mothers and children (62.4%); whereas the haplotypes 677C-1298A and 677C-1298C were predicted at lower frequencies. The rare 677T-1298C haplotype was not predicted for our population. In covariate-adjusted regression models, children of mothers with the 677T-1298A haplotype had lower MDI-24 scores than did children of mothers with the 677C-1298A haplotype (89.9 ± 0.8 compared with 93.3 ± 1.2; P = 0.02). No significant associations were shown between child MTHFR haplotypes and MDI-24 scores.
TABLE 5.
MTHFR haplotype frequencies in relation to Bayley Mental Development Index at 24 mo of age (MDI-24) scores
| Haplotype | Values | Unadjusted MDI-24 scores | Adjusted MDI-24 scores1 |
| Mothers | |||
| 677C-1298A | 27.8 (142)2 | 93.2 ± 1.23 | 93.3 ± 1.2 |
| 677C-1298C | 9.8 (50) | 94.6 ± 2.0 | 93.4 ± 2.1 |
| 677T-1298A | 62.4 (318) | 90.4 ± 0.845 | 89.9 ± 0.84 |
| Children | |||
| 677C-1298A | 26.1 (133) | 92.9 ± 1.3 | 92.6 ± 1.2 |
| 677C-1298C | 11.6 (59) | 91.7 ± 1.8 | 91.4 ± 1.8 |
| 677T-1298A | 62.4 (318) | 91.0 ± 0.8 | 90.5 ± 0.8 |
Models adjusted for maternal age, intelligence quotient, low folate intake, gestational age, parity, marital status, and umbilical cord blood lead.
Percentage; n in parentheses (all such values).
Mean ± SE (all such values).
677C-1298A compared with 677T-1298A (P < 0.05).
677C-1298 compared with C677T-1298A (P < 0.05).
DISCUSSION
In the current study, we observed that increased copies of the maternal MTHFR 677T variant allele were associated with lower child neurodevelopment at 24 mo. The maternal MTHFR haplotype 677T-1298A was also associated with lower neurodevelopment compared with other predicted haplotypes. However, child MTHFR 677 genotypes and haplotypes were not associated with child neurodevelopment, which suggests that the effect, if not because of chance, must be related to maternal-fetal metabolism of folate in pregnancy. Finally, maternal and child MTHFR 1298 genotype status was not associated with child neurodevelopment. In this population there is a high prevalence of MTHFR 677 TT and a low prevalence of MTHFR 1298 CC genotypes as previously reported for populations of Mexican descent (19).
To our knowledge, this is the first study to find a main effect of maternal MTHFR 677 genotype status on child neurodevelopment. The finding that maternal genotypes but not child genotypes are related to child neurodevelopmental outcomes suggests that this effect is due to developmental processes that occur during pregnancy such that maternal genetic variations in folate metabolism may program the trajectories of offspring neurodevelopment.
MTHFR domains/activity
Optimal dietary intake of folate, vitamin B-12, choline, and methionine during pregnancy is necessary for proper fetal development, especially neurogenesis (38). Together, these nutrients are essential components of one-carbon metabolism-dependent nucleotide synthesis and biomethylation reactions. MTHFR is an essential flavoprotein of one-carbon metabolism that catalyzes the NADPH-linked reduction of 5,10-methyleneTHF to 5-methyTHF; the latter provides a methyl group for the de novo generation of methionine from homocysteine for downstream biomethylation reactions (39). The human MTHFR is a dimer of ≈70-kDa subunits; each monomer is composed of both an N-terminal catalytic domain that binds folate and the FAD cofactor and a C-terminal regulatory domain that binds the allosteric inhibitor S-adenosylmethionine (SAM) (13). The 2 monomers are thought to associate in a “head to tail” confirmation to form the catalytically active enzyme dimer (40). The MTHFR 677 and 1298 polymorphic sites are harbored within the catalytic and regulatory domains, respectively. The 677 TT variants have been reported as thermolabile with reduced enzyme activity (12, 13), whereas the 1298 CC variants are associated with only a mild reduction of enzymatic activity in vivo (14). Together, our genotype and haplotype data indicate that the maternal polymorphism located within the catalytic domain (eg, MTHFR 677) may play an important role in child neurodevelopment.
MTHFR prevalence and implications
The prevalence of the MTHFR 677 and 1298 variants has a wide geographic and ethnic variability. It has been reported that Mexico populations have one of the highest frequency of the 677T allele worldwide, whereas they have the lowest frequency of the 1298C allele (19). Our results are consistent with these reports; we showed that >85% of subjects in our study had at least one 677T allele, and > 38% of subjects were TT carriers. In our study, the 1298 CC genotype was only present in ≈1% of subjects.
A well-known interaction exists between the MTHFR 677 genotype and folate status such that a reduction of MTHFR activity occurs in TT carriers under conditions of low but not high folate status. Recombinant studies have shown that lower folate concentrations increase the propensity for MTHFR to lose its FAD cofactor and dissociate into monomers (13). The downstream biological consequences of the MTHFR 677 TT × low folate interaction include elevated plasma homocysteine concentrations (15, 16) and decreased genomic DNA methylation concentrations (17). The high prevalence of the MTHFR 677T allele and low folate intake in our study imply that a reduction of MTHFR activity would be likely. del Río Garcia et al (41) reported a significant interaction between low maternal folate intake (<400 μg/d) and the maternal MTHFR 677 TT genotype on child neurodevelopment. However, in our study we observed no significant interactions between the MTHFR 677 TT genotype and low folate intake. The discrepancy between studies could be explained by different units of measure and cutoffs for low folate intake. First, we converted all folate values to DFEs to account for the increased bioavailability of folic acid derived from supplements. We also used the EAR for pregnant women (520 DFEs/d) to define low folate intake, which resulted in the majority of mothers (83.4%) being classified as having low folate intake. The high prevalence of low folate intake in our population might have limited the statistical power necessary to detect interactions. Finally, although both studies were conducted in Mexico City, our study included measures of lead biomarkers, which increased the precision in estimating the effect of MTHFR genotypes on cognition.
It is important to note that most nutrient components of one-carbon metabolism are actively transported across the placenta against a concentration gradient; for example, folate concentrations are typically 3 times higher in the umbilical cord than in maternal blood (42, 43). Studies have shown that the MTHFR 677 TT genotype is associated with reduced plasma and red cell folate concentrations (15–17) and an altered distribution of red blood cell folate isoforms, where 5,10-methyleneTHF is more prevalent than 5-methylTHF (18). Although the maternal-to-fetal transfer of folate is mediated by the placental folate receptor, which preferentially binds 5-methylTHF isoforms (44), it is unknown to what extent maternal MTHFR 677 genotypes influence the transfer of folate and/or its isoforms across the placental barrier. Shifts in the distribution of folate isoforms in the developing fetus could affect downstream biomethylation reactions or nucleotide synthesis. As in the case of the diomethylation reactions, these could have important implications for the developing nervous system in light of the key role of epigenetic mechanisms, such as DNA and histone methylation, related to neuronal function and memory formation (45).
Lead/cognition
Studies on the effect of nutrition on lead-induced neurocognitive deficits, especially concerning one-carbon metabolism, are underrepresented. SAM, the universal methyl donor for one-carbon metabolism, has been shown to be beneficial in the treatment of lead intoxication by enhancing thiol contents and glutathione concentrations (46). In addition, a recent study revealed that the injection of SAM at postnatal day 22 improved hippocampal long-term potentiation and water-maze performance in rats with lead exposure in early life (47).
In humans, Solon et al (20) showed that folate and/or iron deficiency increased the susceptibility to the negative cognitive effects of lead in children in the central Philippines. Previous work from our group showed that prenatal lead exposure was a significant predictor of poorer child neurodevelopment (7). In the current study, we extend these findings by reporting that the maternal MTHFR 677 TT genotype is also an independent predictor of poorer child neurodevelopment. However, we showed no significant interactions between MTHFR genotypes and lead measures.
A limitation to this study was the use of the semiquantitative FFQ to assess dietary intake. Maternal dietary intake was assessed 1 mo postpartum and was designed to capture dietary habits of women over the previous year (27). Despite the well-know interaction between MTHFR 677 TT carriers and folate deficiency, we did not observe a significant interaction between maternal MTHFR 677 TT carriers and low folate intake on child neurodevelopment. This may be explained, in part, from estimating folate intake from the FFQ rather than assessing folate intake directly through blood measures.
In conclusion, we report that the maternal MTHFR 677T allele is an independent predictor of child neurodevelopment at 24 mo. Our results indicate that maternal genes that influence folate metabolism may influence neurodevelopment trajectories, possibly through fetal programming during pregnancy. Maternal MTHFR genotypes may have important implications on prospective child neurodevelopment in populations that carry a high prevalence of the MTHFR 677T allele, such as in Mexico and areas of China. Additional studies are warranted to determine whether these results are consistent across geographic and/or ethnic groups and to investigate potential molecular pathways that bridge MTHFR genotypes and child neurodevelopment.
Acknowledgments
We thank the field staff and the American British Cowdray Medical Center in Mexico City and the study participants who made this research possible. We also thank Karen Peterson for critical review of the manuscript and insightful comments.
The authors' responsibilities were as follows—JRP: manuscript preparation, statistical analyses, and interpretation of results; HH, ROW, ASE, AC, MMT-R, KK, and MH-A: study design and assistance in the interpretation of results; BNS and DC: assistance in statistical analyses and in interpretation of results; ALL: haplotype analysis; and LS: cognition measurements. None of the authors declared personal or financial conflicts of interest.
REFERENCES
- 1.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr 2001;4:611–24 [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 1989;2:577–80 [DOI] [PubMed] [Google Scholar]
- 3.Rodier PM. Environmental causes of central nervous system maldevelopment. Pediatrics 2004;113:1076–83 [PubMed] [Google Scholar]
- 4.Altmann L, Weinsberg F, Sveinsson K, Lilienthal H, Wiegand H, Winneke G. Impairment of long-term potentiation and learning following chronic lead exposure. Toxicol Lett 1993;66:105–12 [DOI] [PubMed] [Google Scholar]
- 5.Gilbert ME, Kelly ME, Samsam TE, Goodman JH. Chronic developmental lead exposure reduces neurogenesis in adult rat hippocampus but does not impair spatial learning. Toxicol Sci 2005;86:365–74 [DOI] [PubMed] [Google Scholar]
- 6.Nihei MK, Desmond NL, McGlothan JL, Kuhlmann AC, Guilarte TR. N-methyl-D-aspartate receptor subunit changes are associated with lead-induced deficits of long-term potentiation and spatial learning. Neuroscience 2000;99:233–42 [DOI] [PubMed] [Google Scholar]
- 7.Gomaa A, Hu H, Bellinger D, et al. Maternal bone lead as an independent risk factor for fetal neurotoxicity: a prospective study. Pediatrics 2002;110:110–8 [DOI] [PubMed] [Google Scholar]
- 8.Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N Engl J Med 1987;316:1037–43 [DOI] [PubMed] [Google Scholar]
- 9.Hu H, Tellez-Rojo MM, Bellinger D, et al. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect 2006;114:1730–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stover PJ. Physiology of folate and vitamin B12 in health and disease. Nutr Rev 2004;62:S3–12; discussion S13 [DOI] [PubMed] [Google Scholar]
- 11.Stanger O. Physiology of folic acid in health and disease. Curr Drug Metab 2002;3:211–23 [DOI] [PubMed] [Google Scholar]
- 12.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995;10:111–3 [DOI] [PubMed] [Google Scholar]
- 13.Yamada K, Chen Z, Rozen R, Matthews RG. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc Natl Acad Sci USA 2001;98:14853–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Put NM, Gabreels F, Stevens EM, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet 1998;62:1044–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacques PF, Bostom AG, Williams RR, et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation 1996;93:7–9 [DOI] [PubMed] [Google Scholar]
- 16.Girelli D, Friso S, Trabetti E, et al. Methylenetetrahydrofolate reductase C677T mutation, plasma homocysteine, and folate in subjects from northern Italy with or without angiographically documented severe coronary atherosclerotic disease: evidence for an important genetic-environmental interaction. Blood 1998;91:4158–63 [PubMed] [Google Scholar]
- 17.Friso S, Choi SW, Girelli D, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA 2002;99:5606–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagley PJ, Selhub J. A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci USA 1998;95:13217–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gueant-Rodriguez RM, Gueant JL, Debard R, et al. Prevalence of methylenetetrahydrofolate reductase 677T and 1298C alleles and folate status: a comparative study in Mexican, West African, and European populations. Am J Clin Nutr 2006;83:701–7 [DOI] [PubMed] [Google Scholar]
- 20.Solon O, Riddell TJ, Quimbo SA, et al. Associations between cognitive function, blood lead concentration, and nutrition among children in the central Philippines. J Pediatr 2008;152:237–43 [DOI] [PubMed] [Google Scholar]
- 21.Andrews KW, Savitz DA, Hertz-Picciotto I. Prenatal lead exposure in relation to gestational age and birth weight: a review of epidemiologic studies. Am J Ind Med 1994;26:13–32 [DOI] [PubMed] [Google Scholar]
- 22.Kim R, Aro A, Rotnitzky A, Amarasiriwardena C, Hu H. K x-ray fluorescence measurements of bone lead concentration: the analysis of low-level data. Phys Med Biol 1995;40:1475–85 [DOI] [PubMed] [Google Scholar]
- 23.Wigg NR, Vimpani GV, McMichael AJ, Baghurst PA, Robertson EF, Roberts RJ. Port Pirie Cohort study: childhood blood lead and neuropsychological development at age two years. J Epidemiol Community Health 1988;42:213–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasserman G, Graziano JH, Factor-Litvak P, et al. Independent effects of lead exposure and iron deficiency anemia on developmental outcome at age 2 years. J Pediatr 1992;121:695–703 [DOI] [PubMed] [Google Scholar]
- 25.Baghurst PA, Robertson EF, Oldfield RK, et al. Lead in the placenta, membranes, and umbilical cord in relation to pregnancy outcome in a lead-smelter community. Environ Health Perspect 1991;90:315–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothenberg SJ, Schnaas L, Cansino-Ortiz S, et al. Neurobehavioral deficits after low level lead exposure in neonates: the Mexico City pilot study. Neurotoxicol Teratol 1989;11:85–93 [DOI] [PubMed] [Google Scholar]
- 27.Kordas K, Ettinger AS, Lamadrid-Figueroa H, et al. Methylenetetrahydrofolate reductase (MTHFR) C677T, A1298C and G1793A genotypes, and the relationship between maternal folate intake, tibia lead and infant size at birth. Br J Nutr 2009;102:907–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 29.Willett WC, Reynolds RD, Cottrell-Hoehner S, Sampson L, Browne ML. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc 1987;87:43–7 [PubMed] [Google Scholar]
- 30.Romieu I, Hernandez-Avila M, Rivera JA, Ruel MT, Parra S. Dietary studies in countries experiencing a health transition: Mexico and Central America. Am J Clin Nutr 1997;65:1159S–65S [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Avila M, Romieu I, Parra S, Hernandez-Avila J, Madrigal H, Willett W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex 1998;40:133–40 [DOI] [PubMed] [Google Scholar]
- 32.Bailey LB. New standard for dietary folate intake in pregnant women. Am J Clin Nutr 2000;71:1304S–7S [DOI] [PubMed] [Google Scholar]
- 33.Suitor CW, Bailey LB. Dietary folate equivalents: interpretation and application. J Am Diet Assoc 2000;100:88–94 [DOI] [PubMed] [Google Scholar]
- 34.Food and Nutrition Board, Institute of Medicine Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press, 1998 [PubMed] [Google Scholar]
- 35.Mejia-Rodriguez F, Sotres-Alvarez D, Neufeld LM, Garcia-Guerra A, Hotz C. Use of nutritional supplements among Mexican women and the estimated impact on dietary intakes below the EAR and above the UL. J Am Coll Nutr 2007;26:16–23 [DOI] [PubMed] [Google Scholar]
- 36.Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genet 2005;76:449–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 2001;68:978–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeisel SH. Importance of methyl donors during reproduction. Am J Clin Nutr 2009;89:673S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green JM, Ballou DP, Matthews RG. Examination of the role of methylenetetrahydrofolate reductase in incorporation of methyltetrahydrofolate into cellular metabolism. FASEB J 1988;2:42–7 [DOI] [PubMed] [Google Scholar]
- 40.Ulvik A, Ueland PM, Fredriksen A, et al. Functional inference of the methylenetetrahydrofolate reductase 677C > T and 1298A > C polymorphisms from a large-scale epidemiological study. Hum Genet 2007;121:57–64 [DOI] [PubMed] [Google Scholar]
- 41.del Rio Garcia C, Torres-Sanchez L, Chen J, et al. Maternal MTHFR 677C>T genotype and dietary intake of folate and vitamin B(12): their impact on child neurodevelopment. Nutr Neurosci 2009;12:13–20 [DOI] [PubMed] [Google Scholar]
- 42.Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr 2006;83:993–1016 [DOI] [PubMed] [Google Scholar]
- 43.Hall M, Gamble M, Slavkovich V, et al. Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ Health Perspect 2007;115:1503–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antony AC. In utero physiology: role of folic acid in nutrient delivery and fetal development. Am J Clin Nutr 2007;85:598S–603S [DOI] [PubMed] [Google Scholar]
- 45.Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci 2005;6:108–18 [DOI] [PubMed] [Google Scholar]
- 46.Paredes SR, Fukuda H, Kozicki PA, Rossetti MV, Conti H, Batlle AM. S-adenosyl-L-methionine and lead intoxication: its therapeutic effect varying the route of administration. Ecotoxicol Environ Saf 1986;12:252–60 [DOI] [PubMed] [Google Scholar]
- 47.Cao XJ, Huang SH, Wang M, Chen JT, Ruan DY. S-adenosyl-L-methionine improves impaired hippocampal long-term potentiation and water maze performance induced by developmental lead exposure in rats. Eur J Pharmacol 2008;595:30–4 [DOI] [PubMed] [Google Scholar]