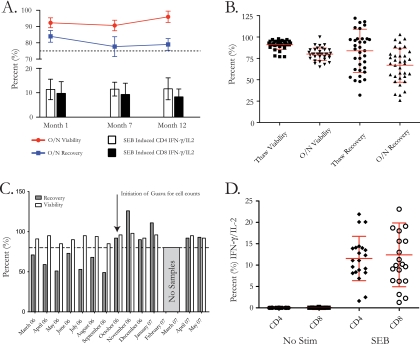
FIG. 3.
The Makerere University Walter Reed Project laboratory follows standard procedures for assessing quality of PBMC collected and cryopreserved during protocol vaccine trials, including internal assessment of longevity, precision, and functionality. (A) PBMC from 3 subjects were assessed for the impact of cryopreservation on recovery, viability, and functionality by thawing the same samples at 1, 7, and 12 months postcryopreservation. The bar and line charts show the means and standard deviations for overnight viability (red), overnight recovery (blue), and control positive responses (IFN-γ/IL-2) to SEB for CD4 (white bars) and CD8 (black bars) cells. (B) Thirty-five samples were processed, cryopreserved (for approximately 1 month), thawed, and assessed for recovery and viability after thawing and overnight rest as part of a monthly internal quality assurance procedure. The scatter plot shows the cumulative mean and standard deviation for all 35 samples. (C) Bar chart showing the mean and standard deviation for thaw recovery and viability for the 35 samples monitored, by month (no samples were processed in February and March 2007). (D) Thirty-five samples were thawed and assessed for function after thaw and overnight rest in response to SEB or in the absence of stimulation. The scatter plot shows the cumulative mean and standard deviation for all 35 samples for CD4 T cells and CD8 T cells.