Abstract
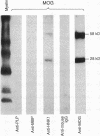
Although T cell responses to the quantitatively major myelin proteins, myelin basic protein (MBP) and proteolipid protein (PLP), are likely to be of importance in the course of multiple sclerosis (MS), cell-mediated autoimmune responses to other myelin antigens, in particular quantitatively minor myelin antigens, such as myelin-associated glycoprotein (MAG) and the central nervous system-specific myelin oligodendrocyte glycoprotein (MOG), could also play a prevalent role in disease initiation or progression. Highly purified myelin antigens were used in this study to assess cell-mediated immune response to MOG in MS patients, in the context of the reactivity to other myelin antigens, MBP, PLP, and MAG. The greatest incidence of proliferative response by MS peripheral blood lymphocytes was to MOG, as 12 of 24 patients tested reacted and, of these, 8 reacted to MOG exclusively. In contrast, only 1 control individual of 16 tested reacted positively to MOG. The incidence of responses to MBP, PLP, and MAG did not differ greatly between MS patients and control individuals. A predominant T cell reactivity to MOG in MS suggests an important role for cell-mediated immune response to this antigen in the pathogenesis of MS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo S., Bernard C. C., Webb M., Johns T. G., Alafaci A., Ward L. D., Simpson R. J., Kerlero de Rosbo N. Preparation of highly purified human myelin oligodendrocyte glycoprotein in quantities sufficient for encephalitogenicity and immunogenicity studies. Biochem Mol Biol Int. 1993 Aug;30(5):945–958. [PubMed] [Google Scholar]
- Amiguet P., Gardinier M. V., Zanetta J. P., Matthieu J. M. Purification and partial structural and functional characterization of mouse myelin/oligodendrocyte glycoprotein. J Neurochem. 1992 May;58(5):1676–1682. doi: 10.1111/j.1471-4159.1992.tb10040.x. [DOI] [PubMed] [Google Scholar]
- Baxevanis C. N., Reclos G. J., Servis C., Anastasopoulos E., Arsenis P., Katsiyiannis A., Matikas N., Lambris J. D., Papamichail M. Peptides of myelin basic protein stimulate T lymphocytes from patients with multiple sclerosis. J Neuroimmunol. 1989 Mar;22(1):23–30. doi: 10.1016/0165-5728(89)90005-2. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Cohen I. R. Experimental autoimmune encephalomyelitis (EAE) mediated by T cell lines: process of selection of lines and characterization of the cells. J Immunol. 1982 Jul;129(1):303–308. [PubMed] [Google Scholar]
- Ben-Nun A., Wekerle H., Cohen I. R. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981 Mar;11(3):195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- Bizzozero O., Besio-Moreno M., Pasquini J. M., Soto E. F., Gómez C. J. Rapid purification of proteolipids from rat brain subcellular fractions by chromatography on a lipophilic dextran gel. J Chromatogr. 1982 Jan 8;227(1):33–44. doi: 10.1016/s0378-4347(00)80353-9. [DOI] [PubMed] [Google Scholar]
- Booss J., Esiri M. M., Tourtellotte W. W., Mason D. Y. Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J Neurol Sci. 1983 Dec;62(1-3):219–232. doi: 10.1016/0022-510x(83)90201-0. [DOI] [PubMed] [Google Scholar]
- Brady R. O., Quarles R. H. Developmental and pathophysiological aspects of the myelin-associated glycoprotein. Cell Mol Neurobiol. 1988 Jun;8(2):139–148. doi: 10.1007/BF00711240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger D., Simon M., Perruisseau G., Steck A. J. The epitope(s) recognized by HNK-1 antibody and IgM paraprotein in neuropathy is present on several N-linked oligosaccharide structures on human P0 and myelin-associated glycoprotein. J Neurochem. 1990 May;54(5):1569–1575. doi: 10.1111/j.1471-4159.1990.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Burger D., Steck A. J., Bernard C. C., Kerlero de Rosbo N. Human myelin/oligodendrocyte glycoprotein: a new member of the L2/HNK-1 family. J Neurochem. 1993 Nov;61(5):1822–1827. doi: 10.1111/j.1471-4159.1993.tb09822.x. [DOI] [PubMed] [Google Scholar]
- Burns J. B., Littlefield K. Human T lymphocytes reactive with whole myelin recognize predominantly myelin basic protein. J Neuroimmunol. 1989 Sep;24(1-2):67–74. doi: 10.1016/0165-5728(89)90100-8. [DOI] [PubMed] [Google Scholar]
- Carnegie P. R., Dowse C. A., Linthicum D. S. Antigenic determinant recognized by a monoclonal antibody to human myelin basic protein. J Neuroimmunol. 1983 Oct;5(2):125–134. doi: 10.1016/0165-5728(83)90003-6. [DOI] [PubMed] [Google Scholar]
- Chou Y. K., Bourdette D. N., Offner H., Whitham R., Wang R. Y., Hashim G. A., Vandenbark A. A. Frequency of T cells specific for myelin basic protein and myelin proteolipid protein in blood and cerebrospinal fluid in multiple sclerosis. J Neuroimmunol. 1992 May;38(1-2):105–113. doi: 10.1016/0165-5728(92)90095-3. [DOI] [PubMed] [Google Scholar]
- Colby S. P., Sheremata W., Bain B., Eylar E. H. Cellular hypersensitivity in attacks of multiple sclerosis. I. A comparative study of migration inhibitory factor production and lymphoblastic transformation in response to myelin basic protein in multiple sclerosis. Neurology. 1977 Feb;27(2):132–139. doi: 10.1212/wnl.27.2.132. [DOI] [PubMed] [Google Scholar]
- Dalakas M. C., Rose J. W., Paul J., Engel W. K., McClure J. E., Goldstein A. L. Increased circulation of T lymphocytes bearing surface thymosin alpha 1 in patients with myasthenia gravis: effect of thymectomy. Neurology. 1983 Feb;33(2):144–149. doi: 10.1212/wnl.33.2.144. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gardinier M. V., Amiguet P., Linington C., Matthieu J. M. Myelin/oligodendrocyte glycoprotein is a unique member of the immunoglobulin superfamily. J Neurosci Res. 1992 Sep;33(1):177–187. doi: 10.1002/jnr.490330123. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Benjamin D. S., Burks J., Weiner H. L. Myelin basic protein and proteolipid protein reactivity of brain- and cerebrospinal fluid-derived T cell clones in multiple sclerosis and postinfectious encephalomyelitis. J Immunol. 1987 Jul 1;139(1):68–72. [PubMed] [Google Scholar]
- Jingwu Z., Medaer R., Hashim G. A., Chin Y., van den Berg-Loonen E., Raus J. C. Myelin basic protein-specific T lymphocytes in multiple sclerosis and controls: precursor frequency, fine specificity, and cytotoxicity. Ann Neurol. 1992 Sep;32(3):330–338. doi: 10.1002/ana.410320305. [DOI] [PubMed] [Google Scholar]
- Johnson D., Hafler D. A., Fallis R. J., Lees M. B., Brady R. O., Quarles R. H., Weiner H. L. Cell-mediated immunity to myelin-associated glycoprotein, proteolipid protein, and myelin basic protein in multiple sclerosis. J Neuroimmunol. 1986 Nov;13(1):99–108. doi: 10.1016/0165-5728(86)90053-6. [DOI] [PubMed] [Google Scholar]
- Kerlero De Rosbo N., Carnegie P. R., Bernard C. C., Linthicum D. S. Detection of various forms of brain myelin basic protein in vertebrates by electroimmunoblotting. Neurochem Res. 1984 Oct;9(10):1359–1369. doi: 10.1007/BF00964663. [DOI] [PubMed] [Google Scholar]
- Kerlero de Rosbo N., Honegger P., Lassmann H., Matthieu J. M. Demyelination induced in aggregating brain cell cultures by a monoclonal antibody against myelin/oligodendrocyte glycoprotein. J Neurochem. 1990 Aug;55(2):583–587. doi: 10.1111/j.1471-4159.1990.tb04173.x. [DOI] [PubMed] [Google Scholar]
- Kuchroo V. K., Sobel R. A., Yamamura T., Greenfield E., Dorf M. E., Lees M. B. Induction of experimental allergic encephalomyelitis by myelin proteolipid-protein-specific T cell clones and synthetic peptides. Pathobiology. 1991;59(5):305–312. doi: 10.1159/000163668. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebar R. Démyélinisation et autoimmunité. Pathol Biol (Paris) 1987 Mar;35(3):275–283. [PubMed] [Google Scholar]
- Lebar R., Lubetzki C., Vincent C., Lombrail P., Boutry J. M. The M2 autoantigen of central nervous system myelin, a glycoprotein present in oligodendrocyte membrane. Clin Exp Immunol. 1986 Nov;66(2):423–434. [PMC free article] [PubMed] [Google Scholar]
- Linington C., Bradl M., Lassmann H., Brunner C., Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988 Mar;130(3):443–454. [PMC free article] [PubMed] [Google Scholar]
- Linington C., Lassmann H. Antibody responses in chronic relapsing experimental allergic encephalomyelitis: correlation of serum demyelinating activity with antibody titre to the myelin/oligodendrocyte glycoprotein (MOG). J Neuroimmunol. 1987 Dec;17(1):61–69. doi: 10.1016/0165-5728(87)90031-2. [DOI] [PubMed] [Google Scholar]
- Link H., Sun J. B., Wang Z., Xu Z., Löve A., Fredrikson S., Olsson T. Virus-reactive and autoreactive T cells are accumulated in cerebrospinal fluid in multiple sclerosis. J Neuroimmunol. 1992 May;38(1-2):63–73. doi: 10.1016/0165-5728(92)90091-x. [DOI] [PubMed] [Google Scholar]
- Linnington C., Webb M., Woodhams P. L. A novel myelin-associated glycoprotein defined by a mouse monoclonal antibody. J Neuroimmunol. 1984 Sep-Oct;6(6):387–396. doi: 10.1016/0165-5728(84)90064-x. [DOI] [PubMed] [Google Scholar]
- Lisak R. P., Zweiman B., Burns J. B., Rostami A., Silberberg D. H. Immune responses to myelin antigens in multiple sclerosis. Ann N Y Acad Sci. 1984;436:221–230. doi: 10.1111/j.1749-6632.1984.tb14793.x. [DOI] [PubMed] [Google Scholar]
- Lisak R. P., Zweiman B., Waters D., Korpowski H., Pleasure D. E. Cell-mediated immunity to measles, myelin basic protein, and central nervous system extract in multiple sclerosis. A longitudinal study employing direct buffy coat migration inhibition assays. Neurology. 1978 Aug;28(8):798–803. doi: 10.1212/wnl.28.8.798. [DOI] [PubMed] [Google Scholar]
- Macklin W. B., Oberfield E., Lees M. B. Electroblot analysis of rat myelin proteolipid protein and basic protein during development. Dev Neurosci. 1983;6(3):161–168. doi: 10.1159/000112343. [DOI] [PubMed] [Google Scholar]
- McCallum K., Esiri M. M., Tourtellotte W. W., Booss J. T cell subsets in multiple sclerosis. Gradients at plaque borders and differences in nonplaque regions. Brain. 1987 Oct;110(Pt 5):1297–1308. doi: 10.1093/brain/110.5.1297. [DOI] [PubMed] [Google Scholar]
- McGarry R. C., Helfand S. L., Quarles R. H., Roder J. C. Recognition of myelin-associated glycoprotein by the monoclonal antibody HNK-1. Nature. 1983 Nov 24;306(5941):376–378. doi: 10.1038/306376a0. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F., McFarlin D. E., Raine C. S. Adoptive transfer of myelin basic protein-sensitized T cells produces chronic relapsing demyelinating disease in mice. Nature. 1984 May 24;309(5966):356–358. doi: 10.1038/309356a0. [DOI] [PubMed] [Google Scholar]
- Ota K., Matsui M., Milford E. L., Mackin G. A., Weiner H. L., Hafler D. A. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990 Jul 12;346(6280):183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- Perry L. L., Barzaga-Gilbert E., Trotter J. L. T cell sensitization to proteolipid protein in myelin basic protein-induced relapsing experimental allergic encephalomyelitis. J Neuroimmunol. 1991 Jul;33(1):7–15. doi: 10.1016/0165-5728(91)90029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser C. M., Paty D. W., Scheinberg L., McDonald W. I., Davis F. A., Ebers G. C., Johnson K. P., Sibley W. A., Silberberg D. H., Tourtellotte W. W. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 Mar;13(3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Raine C. S. Biology of disease. Analysis of autoimmune demyelination: its impact upon multiple sclerosis. Lab Invest. 1984 Jun;50(6):608–635. [PubMed] [Google Scholar]
- Raine C. S. Multiple sclerosis: a pivotal role for the T cell in lesion development. Neuropathol Appl Neurobiol. 1991 Aug;17(4):265–274. doi: 10.1111/j.1365-2990.1991.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Satoh J., Sakai K., Endoh M., Koike F., Kunishita T., Namikawa T., Yamamura T., Tabira T. Experimental allergic encephalomyelitis mediated by murine encephalitogenic T cell lines specific for myelin proteolipid apoprotein. J Immunol. 1987 Jan 1;138(1):179–184. [PubMed] [Google Scholar]
- Schluesener H. J., Sobel R. A., Linington C., Weiner H. L. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol. 1987 Dec 15;139(12):4016–4021. [PubMed] [Google Scholar]
- Sun J. B., Olsson T., Wang W. Z., Xiao B. G., Kostulas V., Fredrikson S., Ekre H. P., Link H. Autoreactive T and B cells responding to myelin proteolipid protein in multiple sclerosis and controls. Eur J Immunol. 1991 Jun;21(6):1461–1468. doi: 10.1002/eji.1830210620. [DOI] [PubMed] [Google Scholar]
- Sun J., Link H., Olsson T., Xiao B. G., Andersson G., Ekre H. P., Linington C., Diener P. T and B cell responses to myelin-oligodendrocyte glycoprotein in multiple sclerosis. J Immunol. 1991 Mar 1;146(5):1490–1495. [PubMed] [Google Scholar]
- Tournier-Lasserve E., Hashim G. A., Bach M. A. Human T-cell response to myelin basic protein in multiple sclerosis patients and healthy subjects. J Neurosci Res. 1988;19(1):149–156. doi: 10.1002/jnr.490190120. [DOI] [PubMed] [Google Scholar]
- Traugott U., Reinherz E. L., Raine C. S. Multiple sclerosis. Distribution of T cells, T cell subsets and Ia-positive macrophages in lesions of different ages. J Neuroimmunol. 1983 Jun;4(3):201–221. doi: 10.1016/0165-5728(83)90036-x. [DOI] [PubMed] [Google Scholar]
- Trifilieff E., Luu B., Nussbaum J. L., Roussel G., Espinosa de los Monteros A., Sabatier J. M., Van Rietschoten J. A specific immunological probe for the major myelin proteolipid. Confirmation of a deletion in DM-20. FEBS Lett. 1986 Mar 31;198(2):235–239. doi: 10.1016/0014-5793(86)80412-4. [DOI] [PubMed] [Google Scholar]
- Trotter J. L., Hickey W. F., van der Veen R. C., Sulze L. Peripheral blood mononuclear cells from multiple sclerosis patients recognize myelin proteolipid protein and selected peptides. J Neuroimmunol. 1991 Jul;33(1):55–62. doi: 10.1016/0165-5728(91)90034-5. [DOI] [PubMed] [Google Scholar]
- Vandenbark A. A., Chou Y. K., Bourdette D., Whitham R., Chilgren J., Chou C. H., Konat G., Hashim G., Vainiene M., Offner H. Human T lymphocyte response to myelin basic protein: selection of T lymphocyte lines from MBP-responsive donors. J Neurosci Res. 1989 May;23(1):21–30. doi: 10.1002/jnr.490230104. [DOI] [PubMed] [Google Scholar]
- Yamamura T., Konola J. T., Wekerle H., Lees M. B. Monoclonal antibodies against myelin proteolipid protein: identification and characterization of two major determinants. J Neurochem. 1991 Nov;57(5):1671–1680. doi: 10.1111/j.1471-4159.1991.tb06367.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Burger D., Saruhan G., Jeannet M., Steck A. J. The T-lymphocyte response against myelin-associated glycoprotein and myelin basic protein in patients with multiple sclerosis. Neurology. 1993 Feb;43(2):403–407. doi: 10.1212/wnl.43.2.403. [DOI] [PubMed] [Google Scholar]
- van der Veen R. C., Trotter J. L., Clark H. B., Kapp J. A. The adoptive transfer of chronic relapsing experimental allergic encephalomyelitis with lymph node cells sensitized to myelin proteolipid protein. J Neuroimmunol. 1989 Feb;21(2-3):183–191. doi: 10.1016/0165-5728(89)90174-4. [DOI] [PubMed] [Google Scholar]