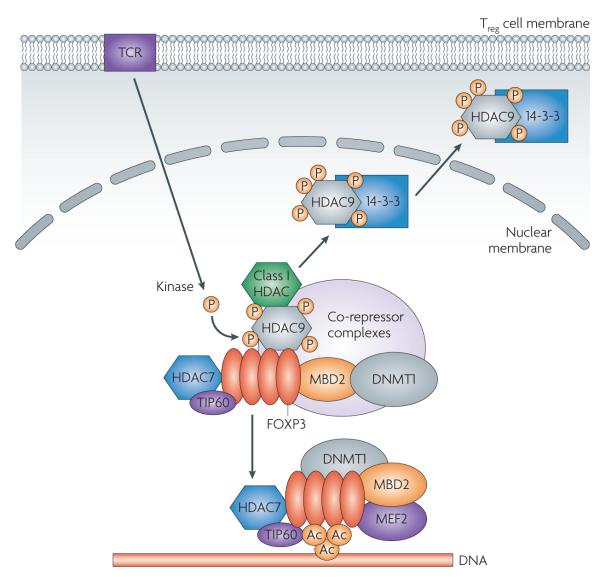
Figure 2. HDAC/FOXP3 complex in regulatory T cells.
Within the nucleus, the forkhead transcription factor FOXP3 undergoes homo- and hetero-oligomerization (the latter with FOXP1) and recruits the histone acetyl transferase (HAT) TIP60 and the class IIa histone deacetylase (HDAC) HDAC7 to a region between 100 and 200 amino acids from the amino-terminus. FOXP3 binding to DNA is impaired by its interaction with a second class IIa HDAC, HDAC9, that acts as a scaffold for assembly of a complex that includes DNA methyltransferase 1 (DNMT1), a class I HDAC (for example, HDAC3), methyl-binding domain 2 (MBD2) and co-repressor complexes, for example, silencing mediator of retinoid and thyroid hormone receptors/nuclear hormone receptor co-repressor (SMRT/NCoR). T cell receptor (TCR) stimulation activates one or more kinases that phosphorylate class II HDACs; candidate kinases include protein kinase D (PKD), calcium/calmodulin-dependent kinase (CaMK) and salt-inducible kinase (SIK). Phosphorylation of HDAC9 acts as an ‘off’ switch, as phosphorylated HDAC9 undergoes a conformational change and dissociates from FOXP3, removing inhibitory complexes and leading to de-repression of FOXP3 and its interaction with other proteins (for example, myocyte enhancing factor 2 (MEF2)) and DNA. Phosphorylated HDAC9 is exported from the nucleus in conjunction with the exportin 14-3-3. In the cytoplasm, phosphorylated HDAC9 is either degraded by the proteasome or might be dephosphorylated and re-enter the nucleus to re-establish binding to FOXP3, limiting its action again (‘on’ switch). Upon removal of HDAC9 and its associated inhibitory complexes, FOXP3 is acetylated by one or more HATs, for example, PCAF and p300, and binds to the promoter regions of target genes in the DNA. Ac denotes acetylation; P denotes phosphorylation.