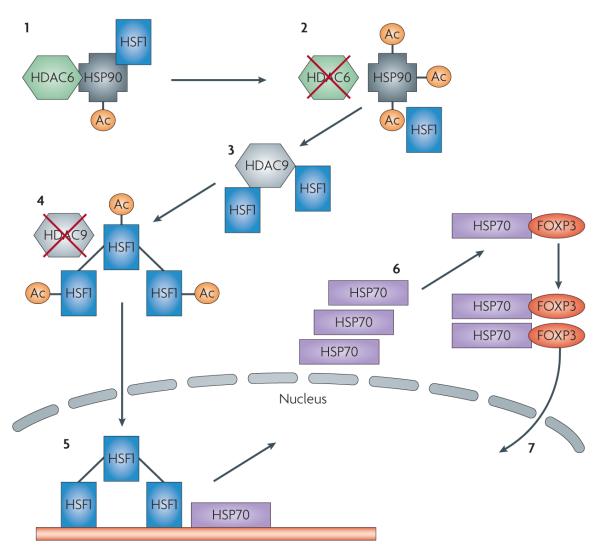
Figure 3. HDAC6 and HDAC9 as synergistic targets for Treg-based histone deacetylase inhibitor (HDACI) therapy.
In the presence of functional HDAC6, heat shock protein 90 (HSP90) exists in a state of basal acetylation and binds multiple client proteins, including heat shock factor HSF1 (1). With inhibition of HDAC6, HSP90 acetylation is markedly enhanced by the actions of one or more unknown histone acetyl transferases and client proteins are released. Whereas many client proteins undergo ubiquitination and proteasomal degradation, others, including HSF1, are functional (2). HDAC9 and SIRT1 may bind HSF1 and differentially maintain its deacetylation (3). Inhibition of HDAC9 promotes HSF1 acetylation, probably by p300, and its trimerization (4). Trimeric HSF1 undergoes phosphorylation by POLO1 or other kinases and nuclear translocation to induce the expression of other heat shock proteins such as HSP70 and HSP27 (5). HSF1-induced HSP70 binds to and serves as a chaperone for many proteins, including FOXP3 (6). HSP70 may promote the maturation of newly synthesized FOXP3 and possibly nuclear translocation and DNA interactions (7). Ac denotes acetylation.