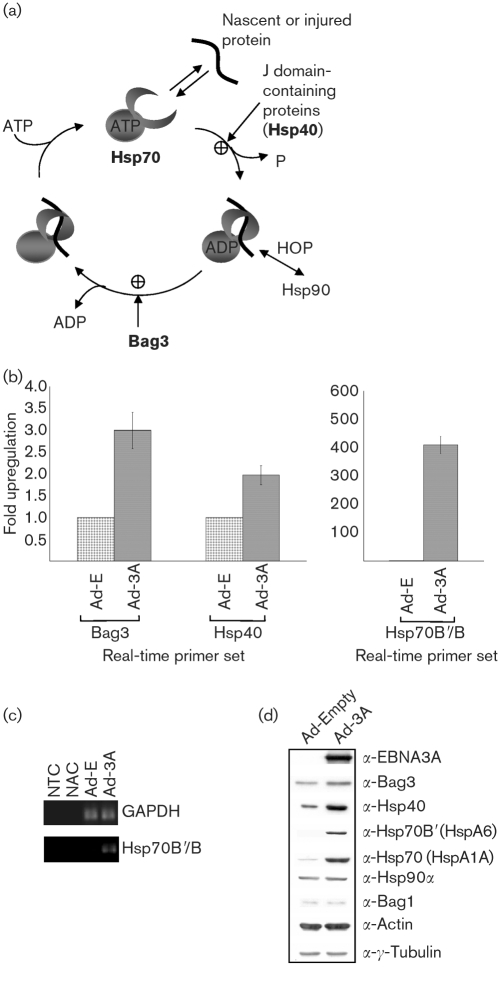
Fig. 2.
EBNA3A induces the co-chaperones necessary for the ATPase cycle of Hsp70. (a) The Hsp70 family is one of the most highly conserved gene families and consists of both constitutive and inducible forms. Hsp70 has a dual function and can either form a complex with a nascent or misfolded protein and chaperone them for refolding, or remove proteins by polyubiquitination and proteasome-mediated degradation (Bukau & Horwich, 1998; Esser et al., 2004). Protein binding by Hsp70 is linked intrinsically to its ATP/ADP-binding status, modulated through the N-terminal ATPase domain (Greene et al., 1995; McCarty et al., 1995; Palleros et al., 1993). Co-chaperones have been identified that can regulate the peptide-binding cycle of Hsp70, by either augmenting or inhibiting protein binding, or by targeting the chaperone to a specific subcellular compartment or protein (reviewed by Mayer, 2005). The co-chaperones Hsp40 and Bag3 (shown in bold) were shown to be upregulated by EBNA3A expression following adenovirus infection of IMR-90 cells. (b) Summary of real-time qRT-PCR analysis using all of the RNA samples from Ad-3A versus Ad-E microarray experiments. GAPDH was run as an endogenous control against which all samples were normalized. (c) End-point real-time qRT-PCR samples were resolved on an agarose gel, demonstrating little to no Hsp70B/B′ DNA product with Ad-E infection. This is consistent with Hsp70B/B′ being silent in most cells. NTC, No template control; NAC, no activation (no reverse transcriptase) control. (d) Western blot analysis of protein samples taken at the time of cell harvesting for the microarray experiments (Table 1). A representative series of blots from one set of protein samples is shown, probed with the antibodies indicated.