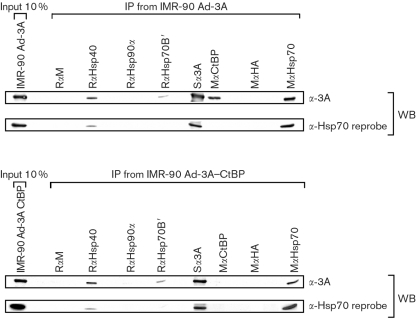
Fig. 4.
EBNA3A binds to Hsp70, Hsp70B′ and the co-chaperone Hsp40 in adenovirus-infected IMR-90 cells. IP from adenovirus-infected IMR-90 cells (m.o.i. of 25, 24 h) using Ad-3A, Ad-3A–CtBP binding mutant and endogenous chaperones and co-chaperones. EBNA3A or EBNA3A–CtBP were co-precipitated with an anti-Hsp70, anti-Hsp70B′ or anti-Hsp40 antibody and detected by Western blot analysis with an anti-EBNA3A antibody. No binding to Hsp90α was observed. Two hundred micrograms of cell lysate was used per IP and all antibodies were used at a 1 : 100 dilution, except anti-CtBP, which was used at a 1 : 200 dilution. Hsp70 antibody was subsequently used to reprobe the membrane and is shown below the respective EBNA3A Western blots. RαM, Rabbit anti-mouse antibody; RαHsp40, rabbit anti-Hsp40; RαHsp90α, rabbit anti-Hsp90α; RαHsp70B′, rabbit anti-Hsp70B′; Sα3A, sheep anti-EBNA3A; RαCtBP, rabbit anti-CtBP; MαHA, mouse anti-haemagglutinin; MαHsp70, mouse anti-Hsp70.