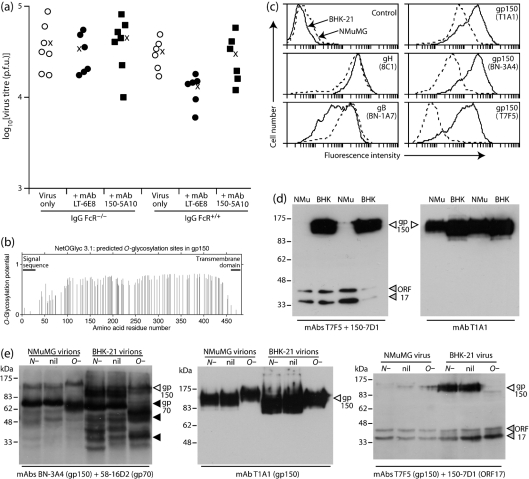
Fig. 7.
O-Glycosylation may make gp150 a poor therapeutic antibody target. (a) C57BL/6 (IgG FcR+/+) or FcRγ−/−FcγRII−/− (IgG FcR−/−) mice were infected i.n. with MuHV-4 (3×104 p.f.u.) and given antibody or not i.p. Five days later, infectious virus in lungs was titrated by plaque assay. Each point shows the titre for one mouse; × shows mean values. IgG FcR+/+ mice showed a significant reduction in virus replication by mAb LT-6E8 (500 μg) (P<0.002), but not by mAb 150-5A10 (200 μg) (P=0.9), using Student's two-tailed t-test. Neither mAb reduced virus replication significantly in IgG FcR−/− mice. (b) Predicted O-glycosylation sites in gp150 (Julenius et al., 2005). The predicted signal sequence (residues 1–22) and transmembrane domain (residues 459–481) are also shown. (c) Flow-cytometric analysis of BHK-21 cells (solid lines) and NMuMG cells (dashed lines) infected with MuHV-4 (2 p.f.u. per cell, 18 h). Control=secondary antibody only. mAbs 8C1 (anti-gH) and BN-1A7 (anti-gB) provide controls for the level of infection. None of the mAbs stained uninfected cells. Staining with mAb 150-5A10 was equivalent to that of BN-3A4. (d) Immunoblot of virions derived from NMuMG cells (NMu) or BHK-21 cells (BHK). Each blot shows two independently grown virus stocks from each cell type. mAb 150-7D1 recognizes the ORF17 capsid component and provides a loading control. (e) Virions derived from NMuMG cells or BHK-21 cells were untreated (nil), digested with PNGase F to remove N-linked glycans (N−) or digested with sialidase+O-glycanase to remove O-linked glycans (O−). Samples were then immunoblotted for gp150. mAb 150-7D1 (ORF17) provides a loading control; mAb 58-16D2 demonstrates O-linked glycan removal from gp70 in the same samples.